Chloroacetyl Chloride on:
[Wikipedia]
[Google]
[Amazon]
Chloroacetyl chloride is a chlorinated acyl chloride. It is a bifunctional compound, making it a useful building block chemical.

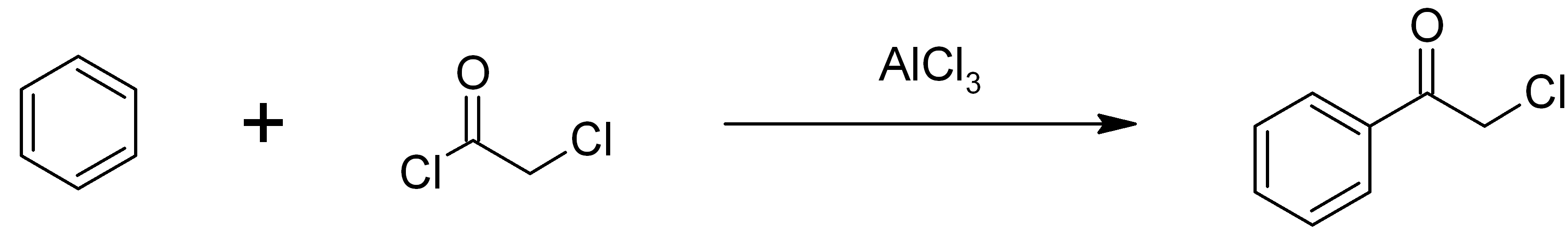 With anisole, it is used for the synthesis of venlafaxine.
With anisole, it is used for the synthesis of venlafaxine.
Production
Industrially, it is produced by thecarbonylation
In chemistry, carbonylation refers to reactions that introduce carbon monoxide (CO) into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemis ...
of methylene chloride, oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
of vinylidene chloride, or the addition of chlorine
Chlorine is a chemical element; it has Symbol (chemistry), symbol Cl and atomic number 17. The second-lightest of the halogens, it appears between fluorine and bromine in the periodic table and its properties are mostly intermediate between ...
to ketene. It may be prepared from chloroacetic acid
Chloroacetic acid, industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula . This carboxylic acid is a useful building block in organic synthesis. It is a colorless solid. Related compounds are dichlo ...
and thionyl chloride
Thionyl chloride is an inorganic compound with the chemical formula . It is a moderately Volatility (chemistry), volatile, colourless liquid with an unpleasant acrid odour. Thionyl chloride is primarily used as a Halogenation, chlorinating reagen ...
, phosphorus pentachloride, or phosgene
Phosgene is an organic chemical compound with the formula . It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. It can be thought of chemically as the double acyl chloride analog of ...
.Reactions
Chloroacetyl chloride is bifunctional—the acyl chloride easily formsester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
s and amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
s, while the other end of the molecule is able to form other linkages, e.g. with amines. The use of chloroacetyl chloride in the synthesis of lidocaine
Lidocaine, also known as lignocaine and sold under the brand name Xylocaine among others, is a local anesthetic of the amino amide type. It is also used to treat ventricular tachycardia and ventricular fibrillation. When used for local anae ...
is illustrative:
:
Applications
The major use of chloroacetyl chloride is as an intermediate in the production of herbicides in the chloroacetanilide family includingmetolachlor
Metolachlor is an organic compound that is widely used as an herbicide. It is a derivative of aniline and is a member of the chloroacetanilide family of herbicides. It is highly effective toward grasses.
Agricultural use
Metolachlor was develo ...
, acetochlor, alachlor
Alachlor is an herbicide from the chloroacetanilide family. It is an odorless, white solid. The greatest use of alachlor is for control of annual grasses and broadleaf weeds in crops. Use of alachlor is illegal in the European Union and no prod ...
and butachlor; an estimated 100 million pounds are used annually. Some chloroacetyl chloride is also used to produce phenacyl chloride, another chemical intermediate, also used as a tear gas. Phenacyl chloride is synthesized in a Friedel-Crafts acylation of benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
, with an aluminium chloride
Aluminium chloride, also known as aluminium trichloride, is an inorganic compound with the formula . It forms a hexahydrate with the formula , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are col ...
catalyst:
: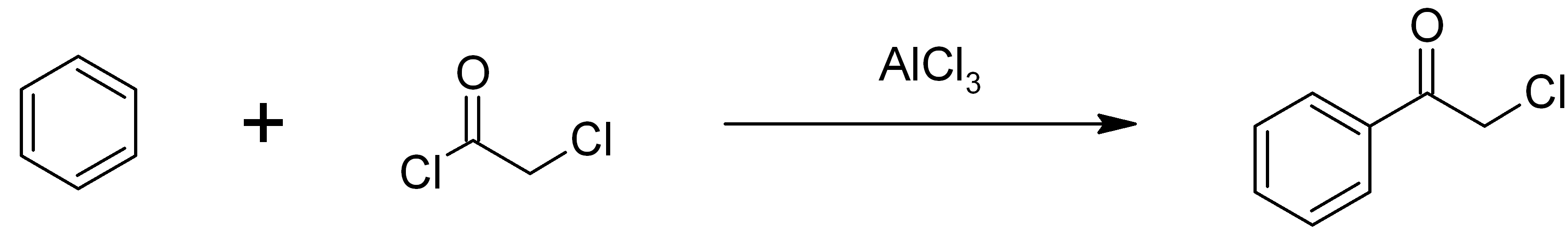 With anisole, it is used for the synthesis of venlafaxine.
With anisole, it is used for the synthesis of venlafaxine.
Safety
Like other acyl chlorides, reaction with other protic compounds such as amines, alcohols, and water generateshydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
, making it a lachrymator.
There is no regulated permissible exposure limit
The permissible exposure limit (PEL or OSHA PEL) is a legal limit in the United States for exposure of an employee to a chemical substance or physical agents such as high level noise. Permissible exposure limits were established by the Occupational ...
set by the Occupational Safety and Health Administration
The Occupational Safety and Health Administration (OSHA; ) is a regulatory agency of the United States Department of Labor that originally had federal visitorial powers to inspect and examine workplaces. The United States Congress established ...
. However, the National Institute for Occupational Safety and Health
The National Institute for Occupational Safety and Health (NIOSH, ) is the List of United States federal agencies, United States federal agency responsible for conducting research and making recommendations for the prevention of work-related occ ...
has set a recommended exposure limit at 0.05 ppm over an eight-hour work day.
References
{{reflist Acyl chlorides Foul-smelling chemicals