|
Ethenone
Ethenone is the formal name for ketene, an organic compound with formula or . It is the simplest member of the ketene class. It is an important reagent for acetylations. Properties Ethenone is a highly reactive gas (at Standard temperature and pressure, standard conditions) and has a sharp irritating odour. It is only reasonably stable at low temperatures (−80 °C). It must therefore always be prepared for each use and processed immediately, otherwise a dimerization to diketene occurs or it reacts to polymers that are difficult to handle. The polymer content formed during the preparation is reduced, for example, by adding sulfur dioxide to the ketene gas. Because of its cumulative double bonds, ethenone is highly reactive and reacts in an addition reaction H-acidic compounds to the corresponding acetic acid derivatives. It does for example react with water to acetic acid or with Primary amine, primary or secondary amines to the corresponding acetamides. Preparation Et ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
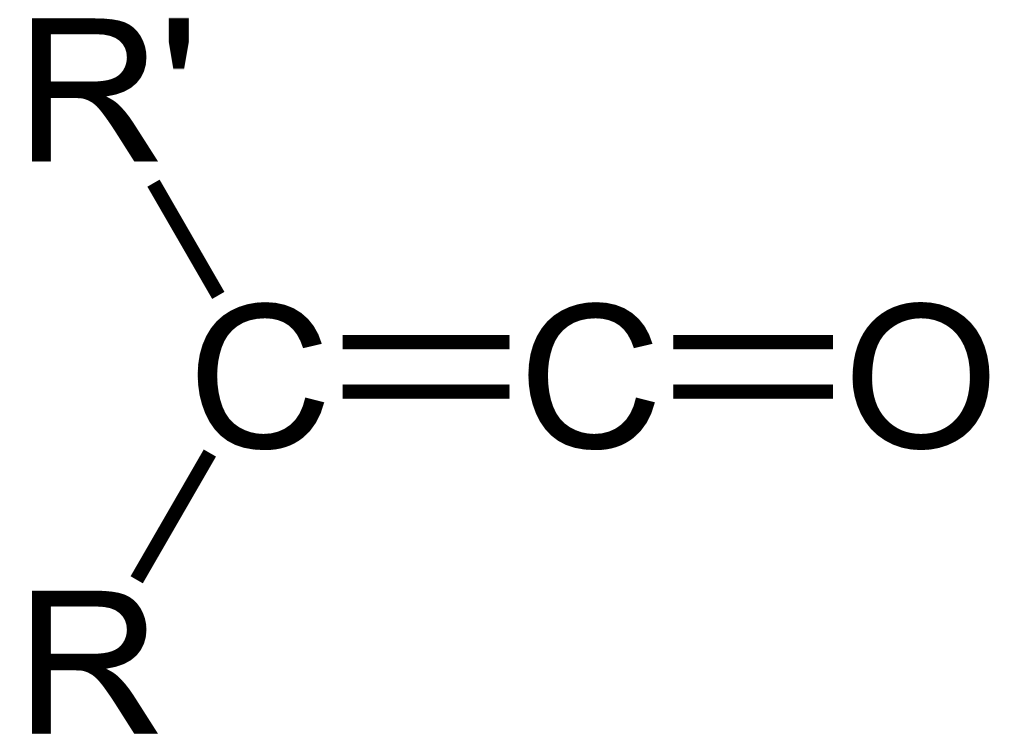
Ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The name may also refer to the specific compound ethenone , the simplest ketene. Although they are highly useful, most ketenes are chemical stability, unstable. When used as reagents in a chemical procedure, they are typically generated when needed, and consumed as soon as (or while) they are produced. History Ketenes were first studied as a class by Hermann Staudinger before 1905. Ketenes were systematically investigated by Hermann Staudinger in 1905 in the form of diphenylketene (conversion of \alpha-chlorodiphenyl acetyl chloride with zinc). Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group). Properties Ketenes are h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diketene
Diketene is an organic compound with the molecular formula , and which is sometimes written as . It is formed by dimerization of ketene, . Diketene is a member of the oxetane family. It is used as a reagent in organic chemistry. It is a colorless liquid. Production Diketene is produced on commercial scale by dimerization of ketene. Reactions Heating or irradiation with UV light regenerates the ketene monomer: : Alkylated ketenes also dimerize with ease and form substituted diketenes. Diketene readily hydrolyzes in water forming acetoacetic acid. Its half-life in water is approximately 45 min. a 25 °C at . Certain diketenes with two aliphatic chains, such as alkyl ketene dimers (AKDs), are used industrially to improve hydrophobicity in paper. At one time acetic anhydride was prepared by the reaction of ketene with acetic acid: : ΔH = −63 kJ/mol Acetoacetylation Diketene also reacts with alcohols and amines to the corresponding acetoacetic acid deri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acetic Anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the chemical formula, formula . Commonly abbreviated , it is one the simplest organic acid anhydride, anhydrides of a carboxylic acid and is widely used in the production of cellulose acetate as well as a reagent in organic synthesis. It is a colorless liquid that smells strongly of acetic acid, which is formed by its reaction with moisture in the air. Structure and properties Acetic anhydride, like most organic acid anhydrides, is a flexible molecule with a nonplanar structure. The C=O and C-O distances are 1.19 and 1.39 Å. The Pi bond, pi system linkage through the central oxygen offers very weak resonance stabilization compared to the dipole, dipole-dipole repulsion between the two carbonyl oxygens. The energy barriers to bond rotation between each of the optimal aplanar conformations are quite low. Production Acetic anhydride was first synthesized in 1852 by the French chemist Charles Frédéric Gerhardt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wiley-VCH
Wiley-VCH is a German publisher owned by John Wiley & Sons. It was founded in 1921 as Verlag Chemie (meaning "Chemistry Press": VCH stands for ''Verlag Chemie'') by two German learned societies A learned society ( ; also scholarly, intellectual, or academic society) is an organization that exists to promote an academic discipline, profession, or a group of related disciplines such as the arts and sciences. Membership may be open to al .... Later, it was merged into the German Chemical Society (GDCh). In 1991, VCH acquired Akademie Verlag. It has been owned by John Wiley & Sons since 1996. The humanities section of Akademie Verlag and the Akademie brand were sold in 1997 to R. Oldenbourg Verlag, while VCH retained the natural sciences catalog. References External links * Wiley (publisher) Publishing companies of Germany Publishing companies established in 1921 Weinheim German companies established in 1921 {{publish-company-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Berichte Der Deutschen Chemischen Gesellschaft
''Chemische Berichte'' (usually abbreviated as ''Ber.'' or ''Chem. Ber.'') was a German-language scientific journal of all disciplines of chemistry founded in 1868. It was one of the oldest scientific journals in chemistry, until it merged with '' Recueil des Travaux Chimiques des Pays-Bas'' to form ''Chemische Berichte/Recueil'' in 1997. ''Chemische Berichte/Recueil'' was then merged with other European journals in 1998 to form '' European Journal of Inorganic Chemistry''. History Founded in 1868 as ''Berichte der Deutschen Chemischen Gesellschaft'' (, CODEN BDCGAS), it operated under this title until 1928 (Vol. 61). The journal was then split into: * ''Berichte der Deutschen Chemischen Gesellschaft, A: Vereins-Nachrichten'' (, CODEN BDCAAS), and * ''Berichte der Deutschen Chemischen Gesellschaft, B: Abhandlungen'' (, CODEN BDCBAD). Vol. 78 and 79 (1945–1946) were omitted and not published due to World War II. The journal was renamed ''Chemische Berichte'' (, CODEN CHBEAM) in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic table. In some respects, zinc is chemically similar to magnesium: both elements exhibit only one normal oxidation state (+2), and the Zn2+ and Mg2+ ions are of similar size. Zinc is the 24th most abundant element in Earth's crust and has five stable isotopes. The most common zinc ore is sphalerite (zinc blende), a zinc sulfide mineral. The largest workable lodes are in Australia, Asia, and the United States. Zinc is refined by froth flotation of the ore, roasting, and final extraction using electricity ( electrowinning). Zinc is an essential trace element for humans, animals, plants and for microorganisms and is necessary for prenatal and postnatal development. It is the second most abundant trace metal in humans after iron, an import ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |