Ethenone on:
[Wikipedia]
[Google]
[Amazon]
In
C2H4O2 -> C2H2O + H2O
On a laboratory scale it can be produced by the thermal decomposition of
 The thermal decomposition of acetic anhydride was also described.
The thermal decomposition of acetic anhydride was also described.



 :
: Ethenone reacts with methanal in the presence of catalysts such as Lewis acids (AlCl3, ZnCl2 or BF3) to give
Ethenone reacts with methanal in the presence of catalysts such as Lewis acids (AlCl3, ZnCl2 or BF3) to give
Ketenes
', 2nd edition. John Wiley & Sons, 2006, .
organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
, ethenone is the formal name for ketene, an organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon- hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
with formula or . It is the simplest member of the ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary monovalent chemical groups (or two separate substitution sites in the same molecule). The name may also refer to the specific compound eth ...
class. It is an important reagent for acetylation
:
In organic chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply ''acetates''. Deacetylation is the opp ...
s.
Properties
Ethenone is a highly reactive gas (atstandard conditions
Standard temperature and pressure (STP) are standard sets of conditions for experimental measurements to be established to allow comparisons to be made between different sets of data. The most used standards are those of the International Union ...
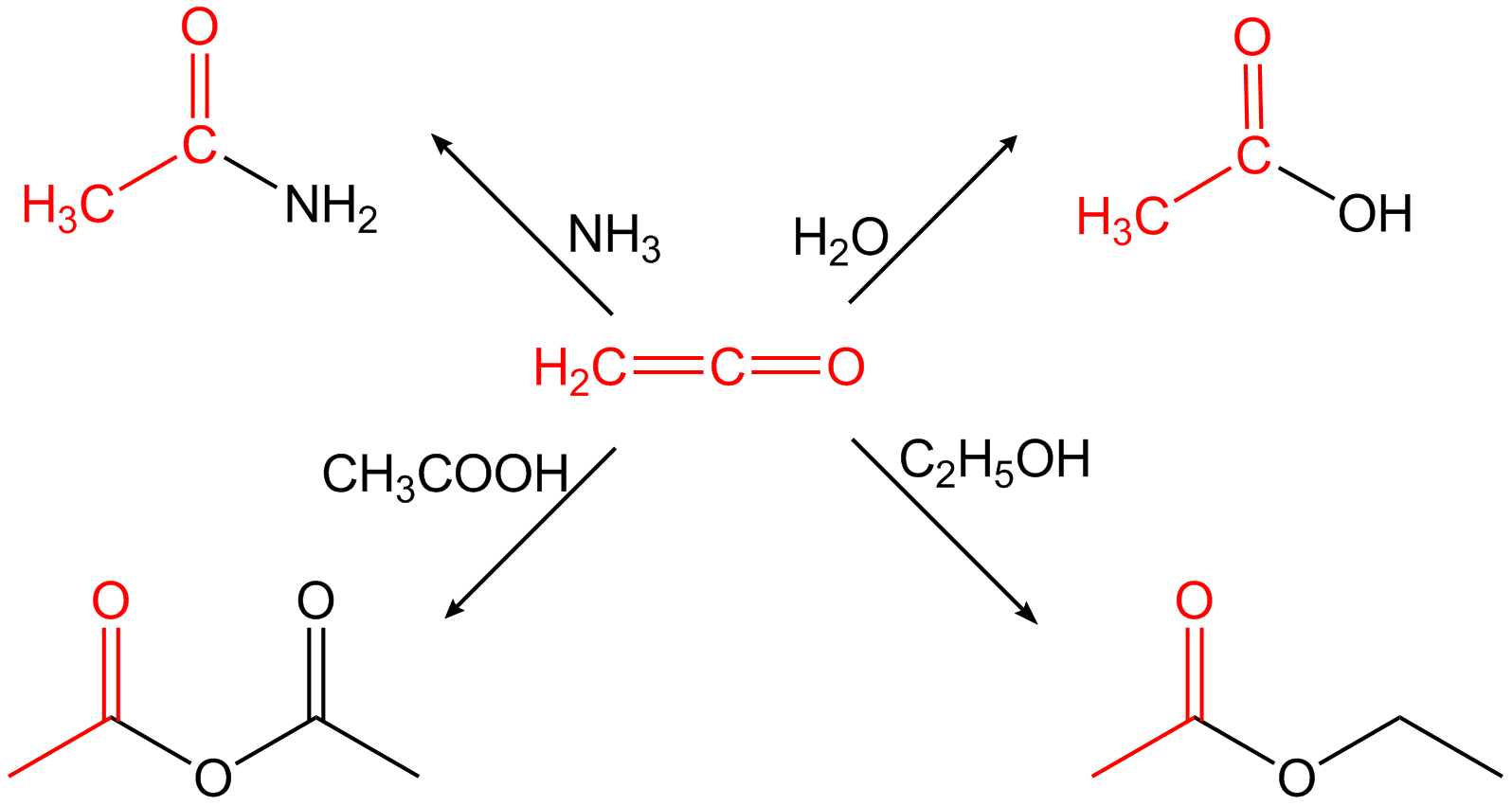
) and has a sharp irritating odour. It is only reasonably stable at low temperatures (−80 °C). It must therefore always be prepared for each use and processed immediately, otherwise a dimerization to diketene occurs or it reacts to polymers that are difficult to handle. The polymer content formed during the preparation is reduced, for example, by adding sulfur dioxide to the ketene gas. Because of its cumulative double bonds, ethenone is highly reactive and reacts in an addition reaction H-acidic compounds to the corresponding acetic acid derivatives. It does for example react with water to acetic acid or with primary
Primary or primaries may refer to:
Arts, entertainment, and media Music Groups and labels
* Primary (band), from Australia
* Primary (musician), hip hop musician and record producer from South Korea
* Primary Music, Israeli record label
Work ...
or secondary amines to the corresponding acetamide
Acetamide (systematic name: ethanamide) is an organic compound with the formula CH3CONH2. It is the simplest amide derived from acetic acid. It finds some use as a plasticizer and as an industrial solvent. The related compound Dimethylacetamide, ...
s.
Preparation
In industrial chemistry, ketene is produced by thedehydration reaction
In chemistry, a dehydration reaction is a chemical reaction that involves the loss of water from the reacting molecule or ion. Dehydration reactions are common processes, the reverse of a hydration reaction.
Dehydration reactions in organic c ...
of acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
:
: Meldrum's acid
Meldrum's acid or 2,2-dimethyl-1,3-dioxane-4,6-dione is an organic compound with formula . Its molecule has a heterocyclic core with four carbon and two oxygen atoms; the formula can also be written as .
It is a crystalline colorless solid, spar ...
at temperatures greater than 200 °C.
History
When passed through heated pipes or electrically heated metal (likecopper
Copper is a chemical element with the symbol Cu (from la, cuprum) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish ...
) wires at 500-600 °C in the presence of carbon disulfide
Carbon disulfide (also spelled as carbon disulphide) is a neurotoxic, colorless, volatile liquid with the formula and structure . The compound is used frequently as a building block in organic chemistry as well as an industrial and chemical ...
, acetone decomposes into methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The relative abundance of methane on Ear ...
and ethenone, with 95% yield.
Ethenone was discovered at the same time by Hermann Staudinger
Hermann Staudinger (; 23 March 1881 – 8 September 1965) was a German organic chemist who demonstrated the existence of macromolecules, which he characterized as polymers. For this work he received the 1953 Nobel Prize in Chemistry.
He is also ...
(by reaction of bromoacetyl bromide with metallic zinc
Zinc is a chemical element with the symbol Zn and atomic number 30. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic t ...
)H. Staudinger H. W. Klever (1908): "Keten. Bemerkung zur Abhandlung zur Abhandlung der HHrn. V.T. Wilsmore und A. W. Stewart". ''Berichte der deutschen chemischen Gesellschaft'', volume 41, issue 1, pages 1516-1517. Tidwell, T. T. (2005), "Ein Jahrhundert Ketene (1905–2005): die Entdeckung einer vielseitigen Klasse reaktiver Intermediate". ''Angewandte Chemie'', volume 117, pages 5926–5933. The dehydration of acetic acid was reported in 1910.
 The thermal decomposition of acetic anhydride was also described.
The thermal decomposition of acetic anhydride was also described.



Natural occurrence
Ethenone has been observed to occur in space, in comets or in gas as part of the interstellar medium.Use
Ethenone is used to makeacetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a c ...
from acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
. Generally it is used for the acetylation
:
In organic chemistry, acetylation is an organic esterification reaction with acetic acid. It introduces an acetyl group into a chemical compound. Such compounds are termed ''acetate esters'' or simply ''acetates''. Deacetylation is the opp ...
of chemical compounds.
: :
:β-propiolactone
β-Propiolactone is an organic compound of the lactone family, with a four-membered ring. It is a colorless liquid with a slightly sweet odor, highly soluble in water and miscible with ethanol, acetone, diethyl ether and chloroform.''Merck Index ...
. The technically most significant use of ethenone is the synthesis of sorbic acid
Sorbic acid, or 2,4-hexadienoic acid, is a natural organic compound used as a food preservative. It has the chemical formula and the structure . It is a colourless solid that is slightly soluble in water and sublimes readily. It was first iso ...
by reaction with 2-butenal
Crotonaldehyde is a chemical compound with the formula CH3CH=CHCHO. The compound is usually sold as a mixture of the ''E''- and ''Z''-isomers, which differ with respect to the relative position of the methyl and formyl groups. The ''E''-isomer ...
(crotonaldehyde) in toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon. It is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) ...
at about 50 °C in the presence of zinc salts of long-chain carboxylic acids. This produces a polyester of 3-hydroxy-4-hexenoic acid, which is thermally or hydrolytically depolymerized to sorbic acid.
Ethenone is very reactive, tending to react with nucleophiles
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
to form an acetyl group
In organic chemistry, acetyl is a functional group with the chemical formula and the structure . It is sometimes represented by the symbol Ac (not to be confused with the element actinium). In IUPAC nomenclature, acetyl is called ethanoyl ...
. For example, it reacts with water to form acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
; with acetic acid to form acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the formula (CH3CO)2O. Commonly abbreviated Ac2O, it is the simplest isolable anhydride of a carboxylic acid and is widely used as a reagent in organic synthesis. It is a c ...
; with ammonia and amines to form ethanamides; and with dry hydrogen halides
In chemistry, hydrogen halides (hydrohalic acids when in the aqueous phase) are diatomic, inorganic compounds that function as Arrhenius acids. The formula is HX where X is one of the halogens: fluorine, chlorine, bromine, iodine, or astatine. A ...
to form acetyl halides.
The formation of acetic acid likely occurs by an initial formation of 1,1-dihydroxyethene, which then tautomerizes to give the final product.
Ethenone will also react with itself via + 2photocycloadditions to form cyclic dimers known as diketenes. For this reason, it should not be stored for long periods.
Hazards
Exposure to concentrated levels causes humans to experience irritation of body parts such as theeye
Eyes are organs of the visual system. They provide living organisms with vision, the ability to receive and process visual detail, as well as enabling several photo response functions that are independent of vision. Eyes detect light and conv ...
, nose
A nose is a protuberance in vertebrates that houses the nostrils, or nares, which receive and expel air for respiration alongside the mouth. Behind the nose are the olfactory mucosa and the sinuses. Behind the nasal cavity, air next pass ...
, throat
In vertebrate anatomy, the throat is the front part of the neck, internally positioned in front of the vertebrae. It contains the pharynx and larynx. An important section of it is the epiglottis, separating the esophagus from the trachea (windpi ...
and lungs. Extended toxicity testing on mice, rats, guinea pigs and rabbits showed that ten-minute exposures to concentrations of freshly generated ethenone as low as 0.2 mg/liter (116 ppm) may produce a high percentage of deaths in small animals. These findings show ethenone is toxicologically identical to phosgene
Phosgene is the organic chemical compound with the formula COCl2. It is a toxic, colorless gas; in low concentrations, its musty odor resembles that of freshly cut hay or grass. Phosgene is a valued and important industrial building block, es ...
.
The formation of ketene in the pyrolysis
The pyrolysis (or devolatilization) process is the thermal decomposition of materials at elevated temperatures, often in an inert atmosphere. It involves a change of chemical composition. The word is coined from the Greek-derived elements ''p ...
of vitamin E acetate, an additive of some e-liquid
An electronic cigarette is a handheld battery-powered vaporizer that simulates smoking, but without tobacco combustion. E-cigarette components include a mouthpiece (drip tip), a cartridge (liquid storage area), a heating element/ atomizer, a m ...
products, is one possible mechanism of the reported pulmonary damage caused by electronic cigarette use.
A number of patents describe the catalytic formation of ketene from carboxylic acids and acetates, using a variety of metals or ceramics, some of which are known to occur in e-cigarette devices from patients with e-cigarette or vaping product-use associated lung injury (EVALI).U.S. patent No. 5475144. Catalyst and process for synthesis of ketenes from carboxylic acids. Dec 12, 1995. https://patents.google.com/patent/US5475144A/en
Occupational exposure limits are set at 0.5 ppm (0.9 mg/m3) over an eight-hour time-weighted average.
An IDLH
The term immediately dangerous to life or health (IDLH) is defined by the US National Institute for Occupational Safety and Health (NIOSH) as exposure to airborne contaminants that is "likely to cause death or immediate or delayed permanent advers ...
limit is set at 5 ppm, as this is the lowest concentration productive of a clinically relevant physiologic response in humans.
References
Literature
* Tidwell, Thomas T.Ketenes
', 2nd edition. John Wiley & Sons, 2006, .
External links
* {{Chemical agents Ketenes Gases Pulmonary agents Acetylating agents