chloride shift on:
[Wikipedia]
[Google]
[Amazon]
 Chloride shift (also known as the Hamburger phenomenon or lineas phenomenon, named after Hartog Jakob Hamburger) is a process which occurs in a
Chloride shift (also known as the Hamburger phenomenon or lineas phenomenon, named after Hartog Jakob Hamburger) is a process which occurs in a
 Chloride shift (also known as the Hamburger phenomenon or lineas phenomenon, named after Hartog Jakob Hamburger) is a process which occurs in a
Chloride shift (also known as the Hamburger phenomenon or lineas phenomenon, named after Hartog Jakob Hamburger) is a process which occurs in a cardiovascular system
In vertebrates, the circulatory system is a system of organs that includes the heart, blood vessels, and blood which is circulated throughout the body. It includes the cardiovascular system, or vascular system, that consists of the heart a ...
and refers to the exchange of bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula .
Bicarbonate serves a crucial bioche ...
(HCO3−) and chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
(Cl−) across the membrane of red blood cell
Red blood cells (RBCs), referred to as erythrocytes (, with -''cyte'' translated as 'cell' in modern usage) in academia and medical publishing, also known as red cells, erythroid cells, and rarely haematids, are the most common type of blood cel ...
s (RBCs).
Mechanism
Carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
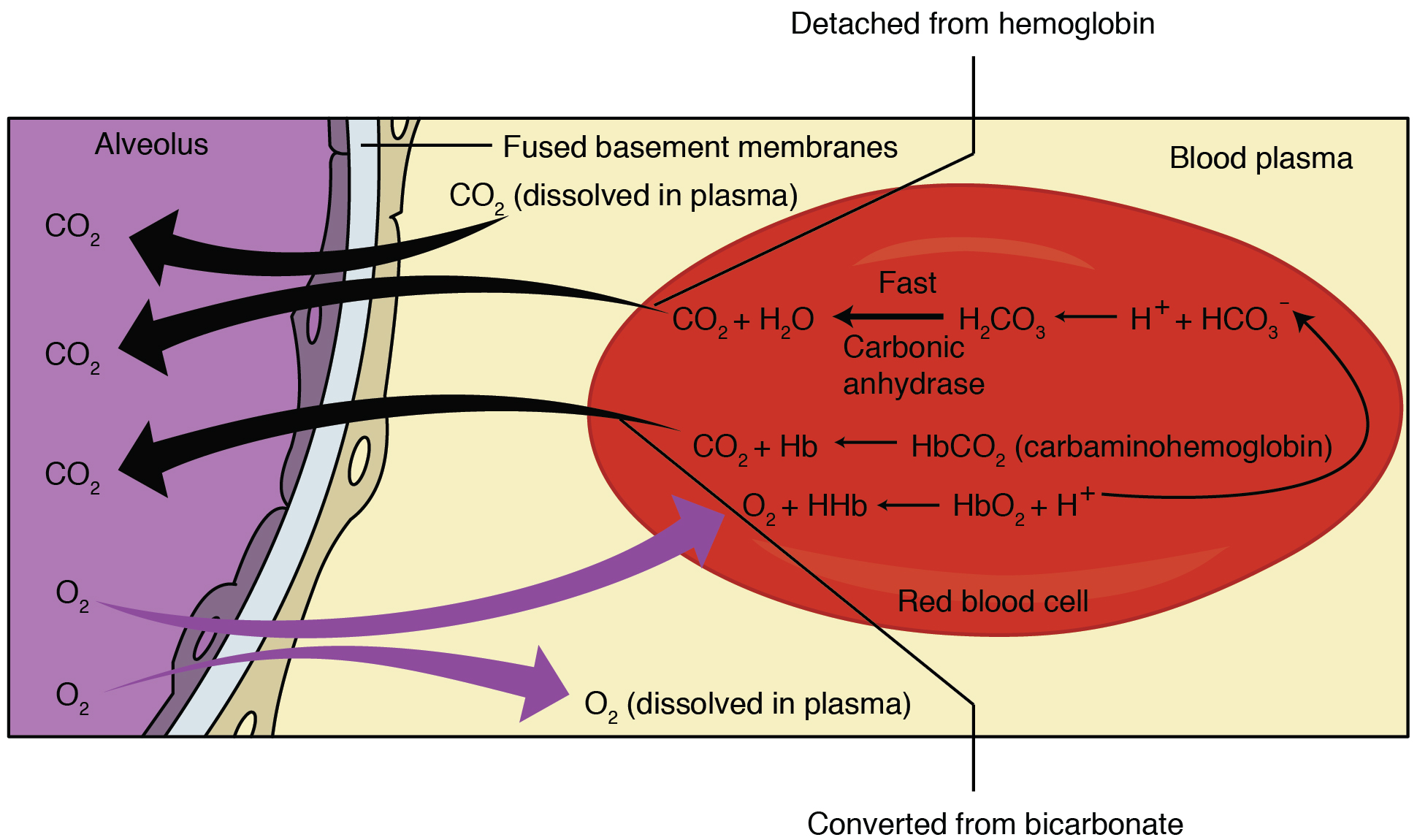
(CO2) is produced in tissues as a byproduct of normal aerobic metabolism. It dissolves in the solution of blood plasma and into red blood cells (RBC), where carbonic anhydrase catalyzes its hydration to carbonic acid
Carbonic acid is a chemical compound with the chemical formula . The molecule rapidly converts to water and carbon dioxide in the presence of water. However, in the absence of water, it is quite stable at room temperature. The interconversion ...
(H2CO3). Carbonic acid then spontaneously dissociate
Dissociation in chemistry is a general process in which molecules (or ionic compounds such as salts, or complexes) separate or split into other things such as atoms, ions, or radicals, usually in a reversible manner. For instance, when an aci ...
s to form bicarbonate Ions (HCO3−) and a hydrogen ion
A hydrogen ion is created when a hydrogen atom loses or gains an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particl ...
(H+). In response to the decrease in intracellular pCO2, more CO2 passively diffuses into the cell.
Cell membranes are generally impermeable to charged ions (i.e. H+, HCO3− ) but RBCs are able to exchange bicarbonate for chloride using the anion exchanger protein Band 3. Thus, the rise in intracellular bicarbonate leads to bicarbonate export and chloride intake. The term "chloride shift" refers to this exchange. Consequently, chloride concentration is lower in systemic venous blood than in systemic arterial blood: high venous pCO2 leads to bicarbonate production in RBCs, which then leaves the RBC in exchange for chloride coming in.
The opposite process occurs in the pulmonary capillaries of the lungs when the PO2 rises and PCO2 falls, and the Haldane effect occurs (release of CO2 from hemoglobin during oxygenation). This releases hydrogen ions from hemoglobin, increases free H+ concentration within RBCs, and shifts the equilibrium towards CO2 and water formation from bicarbonate. The subsequent decrease in intracellular bicarbonate concentration reverses chloride-bicarbonate exchange: bicarbonate moves into the cell in exchange for chloride moving out. Inward movement of bicarbonate via the Band 3 exchanger allows carbonic anhydrase to convert it to CO2 for expiration.
The chloride shift may also regulate the affinity of hemoglobin
Hemoglobin (haemoglobin, Hb or Hgb) is a protein containing iron that facilitates the transportation of oxygen in red blood cells. Almost all vertebrates contain hemoglobin, with the sole exception of the fish family Channichthyidae. Hemoglobin ...
for oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
through the chloride ion acting as an allosteric effector.
Reaction
Reaction (as it occurs in the pulmonary capillaries) RBC PLASMA HCO3− <-- <-- <-- HCO3− K+ Na+ Cl− --> --> --> --> Cl− Bicarbonate in the red blood cell (RBC) exchanging with chloride from plasma in the lungs. The underlying properties creating the chloride shift are the presence of carbonic anhydrase within the RBCs but not the plasma, and the permeability of the RBC membrane to carbon dioxide and bicarbonate ion but not to hydrogen ion. Continuous process of carbonic acid dissociation and outflow of bicarbonate ions would eventually lead to a change of intracellular electric potential because of lasting H+ ions. Inflow of chloride ions maintains electrical neutrality of a cell. The net direction of bicarbonate-chloride exchange (bicarbonate out of RBCs in the systemic capillaries, bicarbonate into RBCs at pulmonary capillaries) proceeds in the direction that decreases the sum of the electrochemical potentials for the chloride and bicarbonate ions being transported.References
{{DEFAULTSORT:Chloride Shift Blood Respiratory physiology