Chiral Auxiliaries on:
[Wikipedia]
[Google]
[Amazon]
In 
Most biological molecules and pharmaceutical targets exist as one of two possible
 A typical auxiliary-guided stereoselective transformation involves three steps: first, the auxiliary is covalently coupled to the substrate; second, the resulting compound undergoes one or more diastereoselective transformations; and finally, the auxiliary is removed under conditions that do not cause racemization of the desired products. The cost of employing stoichiometric auxiliary and the need to spend synthetic steps appending and removing the auxiliary make this approach appear inefficient. However, for many transformations, the only available stereoselective methodology relies on chiral auxiliaries. In addition, transformations with chiral auxiliaries tend to be versatile and very well-studied, allowing the most time-efficient access to enantiomerically pure products.
Furthermore, the products of auxiliary-directed reactions are
A typical auxiliary-guided stereoselective transformation involves three steps: first, the auxiliary is covalently coupled to the substrate; second, the resulting compound undergoes one or more diastereoselective transformations; and finally, the auxiliary is removed under conditions that do not cause racemization of the desired products. The cost of employing stoichiometric auxiliary and the need to spend synthetic steps appending and removing the auxiliary make this approach appear inefficient. However, for many transformations, the only available stereoselective methodology relies on chiral auxiliaries. In addition, transformations with chiral auxiliaries tend to be versatile and very well-studied, allowing the most time-efficient access to enantiomerically pure products.
Furthermore, the products of auxiliary-directed reactions are
 (−)-8-phenylmenthol can be prepared from either
(−)-8-phenylmenthol can be prepared from either
 The preparation of a variety of enantiomerically pure uncommon ''R''-amino acids can be achieved by the alkylation of chiral glycine derivatives possessing axially chiral BINOL as an auxiliary. It has been depicted by Fuji et al. Based on different
The preparation of a variety of enantiomerically pure uncommon ''R''-amino acids can be achieved by the alkylation of chiral glycine derivatives possessing axially chiral BINOL as an auxiliary. It has been depicted by Fuji et al. Based on different  Protected at the
Protected at the  BINOL was also used as a chiral auxiliary to control the formation of a P-stereocenter in an asymmetric metal-catalyzed C-P coupling process. Mondal et al. discovered that the Pd-catalysed C-P cross-coupling reaction between axially chiral BINOL-based phosphoramidites and aryl halides or triflates proceeds with excellent stereoselectivity due to the presence of BINOL near the reacting P center.
BINOL was also used as a chiral auxiliary to control the formation of a P-stereocenter in an asymmetric metal-catalyzed C-P coupling process. Mondal et al. discovered that the Pd-catalysed C-P cross-coupling reaction between axially chiral BINOL-based phosphoramidites and aryl halides or triflates proceeds with excellent stereoselectivity due to the presence of BINOL near the reacting P center.
 One type of chiral auxiliary is based on the ''trans''-2-phenylcyclohexanol motif as introduced by James K. Whitesell and coworkers in 1985. This chiral auxiliary was used in ene reactions of the derived
One type of chiral auxiliary is based on the ''trans''-2-phenylcyclohexanol motif as introduced by James K. Whitesell and coworkers in 1985. This chiral auxiliary was used in ene reactions of the derived  In the total synthesis of (−)-heptemerone B and (−)-guanacastepene E, attached with trans-2-phenylcyclohexanol, the glyoxylate reacted with 2,4-dimethyl-pent-2-ene, in the presence of
In the total synthesis of (−)-heptemerone B and (−)-guanacastepene E, attached with trans-2-phenylcyclohexanol, the glyoxylate reacted with 2,4-dimethyl-pent-2-ene, in the presence of  For even greater conformational control, switching from a
For even greater conformational control, switching from a 
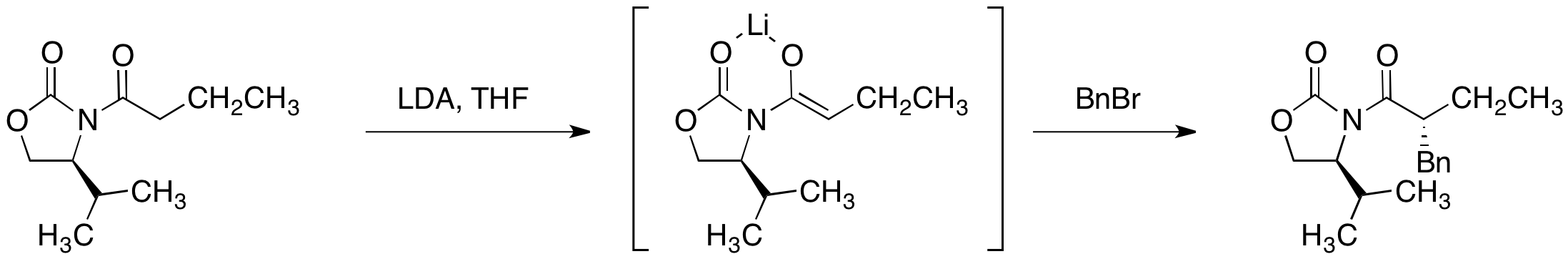
 Acylation of the oxazolidinone is achieved by deprotonation with n-butyllithium and quench with an
Acylation of the oxazolidinone is achieved by deprotonation with n-butyllithium and quench with an 
 Activated electrophiles, such as
Activated electrophiles, such as
 A model for the observed stereoselectivity can be found below. The ''syn''-stereo relationship between the methyl group and the new secondary alcohol results from a six-membered ring Zimmerman-Traxler
A model for the observed stereoselectivity can be found below. The ''syn''-stereo relationship between the methyl group and the new secondary alcohol results from a six-membered ring Zimmerman-Traxler 

 In the total synthesis of manzacidin B, Ohfune group utilized camphorsultam to construct the core
In the total synthesis of manzacidin B, Ohfune group utilized camphorsultam to construct the core  Camphorsultam also acts as a chiral auxiliary in Michael addition. Lithium base promoted stereoselective Michael addition of thiols to N-mcthacryloylcamphorsultam produced the corresponding addition products in high diastereoselectivity.
Camphorsultam also acts as a chiral auxiliary in Michael addition. Lithium base promoted stereoselective Michael addition of thiols to N-mcthacryloylcamphorsultam produced the corresponding addition products in high diastereoselectivity.
 Camphorsultam was used as a chiral auxiliary for the asymmetric
Camphorsultam was used as a chiral auxiliary for the asymmetric 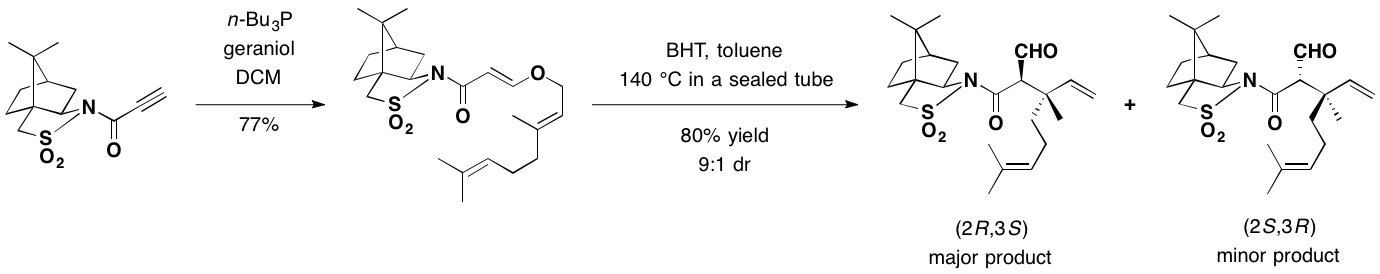
 Pseudoephedrine amides are typically prepared by acylation with an acyl chloride or
Pseudoephedrine amides are typically prepared by acylation with an acyl chloride or 
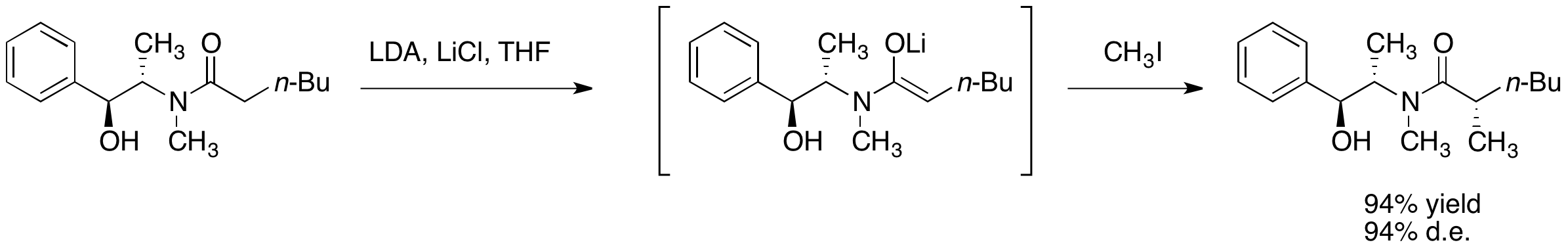
The diastereoselectivity is believed to result from a configuration wherein one face of the lithium enolate is blocked by the secondary lithium alkoxide and the solvent molecules associated with that lithium cation. In accordance with this proposal, it has been observed that the diastereoselectivity of the alkylation step is highly dependent on the amount of lithium chloride present and on the solvent,
One primary advantage of asymmetric alkylation with pseudoephedrine amides is that the amide enolates are typically nucleophilic enough to react with primary and even secondary halides at temperatures ranging from –78 °C to 0 °C. Construction of quaternary carbon centers by alkylation of α-branched amide enolates is also possible, though the addition of DMPU is necessary for less reactive electrophiles.



Condensation of ''tert''-butanesulfinamide with an aldehyde or ketone proceeds in high yield and affords only the (''E'')-isomer of the corresponding ''N''-sulfinyl imines.



stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
, a chiral auxiliary is a stereogenic
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups cr ...
group or unit that is temporarily incorporated into an organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
in order to control the stereochemical outcome of the synthesis. The chirality
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable fro ...
present in the auxiliary can bias the stereoselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation ...
of one or more subsequent reactions. The auxiliary can then be typically recovered for future use.

Most biological molecules and pharmaceutical targets exist as one of two possible
enantiomers
In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities whi ...
; consequently, chemical syntheses of natural products and pharmaceutical agents are frequently designed to obtain the target in enantiomerically pure form. Chiral auxiliaries are one of many strategies available to synthetic chemists to selectively produce the desired stereoisomer of a given compound.
Chiral auxiliaries were introduced by Elias James Corey in 1975 with chiral 8-phenylmenthol and by Barry Trost in 1980 with chiral mandelic acid. The menthol compound is difficult to prepare and as an alternative trans-2-phenyl-1-cyclohexanol was introduced by J. K. Whitesell in 1985.
Asymmetric synthesis
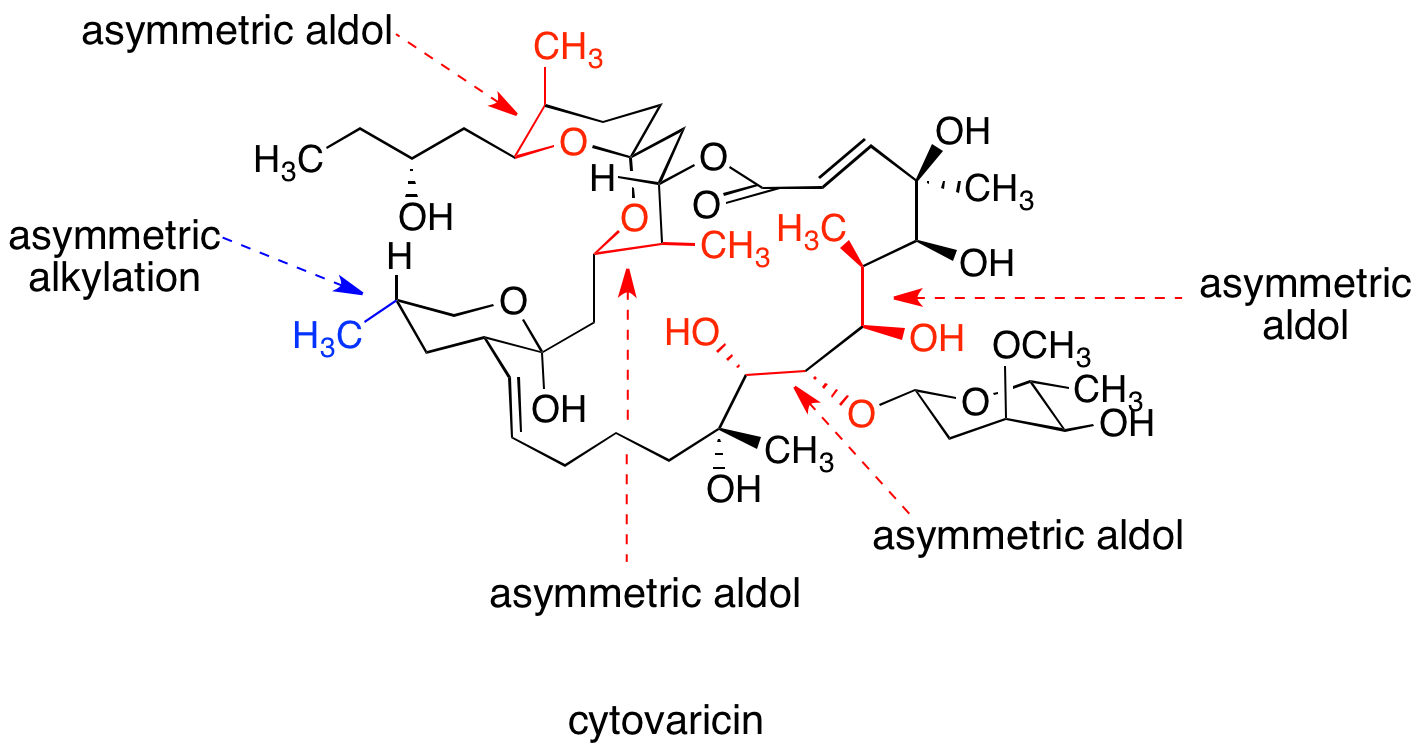
Chiral auxiliaries are incorporated into synthetic routes to control the absolute configuration of stereogenic centers. David A. Evans' synthesis of the macrolide cytovaricin, considered a classic, utilizes oxazolidinone chiral auxiliaries for one asymmetric alkylation reaction and four asymmetric aldol reactions, setting the absolute stereochemistry of nine stereocenters. A typical auxiliary-guided stereoselective transformation involves three steps: first, the auxiliary is covalently coupled to the substrate; second, the resulting compound undergoes one or more diastereoselective transformations; and finally, the auxiliary is removed under conditions that do not cause racemization of the desired products. The cost of employing stoichiometric auxiliary and the need to spend synthetic steps appending and removing the auxiliary make this approach appear inefficient. However, for many transformations, the only available stereoselective methodology relies on chiral auxiliaries. In addition, transformations with chiral auxiliaries tend to be versatile and very well-studied, allowing the most time-efficient access to enantiomerically pure products.
Furthermore, the products of auxiliary-directed reactions are
A typical auxiliary-guided stereoselective transformation involves three steps: first, the auxiliary is covalently coupled to the substrate; second, the resulting compound undergoes one or more diastereoselective transformations; and finally, the auxiliary is removed under conditions that do not cause racemization of the desired products. The cost of employing stoichiometric auxiliary and the need to spend synthetic steps appending and removing the auxiliary make this approach appear inefficient. However, for many transformations, the only available stereoselective methodology relies on chiral auxiliaries. In addition, transformations with chiral auxiliaries tend to be versatile and very well-studied, allowing the most time-efficient access to enantiomerically pure products.
Furthermore, the products of auxiliary-directed reactions are diastereomers
In stereochemistry, diastereomers (sometimes called diastereoisomers) are a type of stereoisomer. Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have dif ...
, which enables their facile separation by methods such as column chromatography or crystallization.
8-phenylmenthol
In an early example of the use of a chiral auxiliary in asymmetric synthesis, E. J. Corey and coworkers conducted an asymmetric Diels-Alder reaction between (−)-8-phenylmentholacrylate
Acrylates (IUPAC: prop-2-enoates) are the salts, esters, and conjugate bases of acrylic acid. The acrylate ion is the anion . Often, acrylate refers to esters of acrylic acid, the most common member being methyl acrylate. These acrylates contain ...
ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
and 5-benzyloxymethylcyclopentadiene. The cycloaddition product was carried forward to the iodolactone shown below, an intermediate in the classic Corey synthesis of the prostaglandins
Prostaglandins (PG) are a group of physiologically active lipid compounds called eicosanoids that have diverse hormone-like effects in animals. Prostaglandins have been found in almost every tissue in humans and other animals. They are derive ...
. It is proposed that the back face of the acrylate is blocked by the auxiliary, so that cycloaddition occurs at the front face of the alkene.
 (−)-8-phenylmenthol can be prepared from either
(−)-8-phenylmenthol can be prepared from either enantiomer
In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities whi ...
of pulegone,
though neither route is very efficient. Because of the widespread utility of the 8-phenylmenthol auxiliary, alternative compounds that are more easily synthesized, such as ''trans''-2-phenyl-1-cyclohexanol
and ''trans''-2-(1-pheyl-1-methylethyl)cyclohexanol have been explored.
1,1’-Binaphthyl-2,2’-diol (BINOL)
1,1’-Binaphthyl-2,2’-diol, or BINOL, has been used as chiral auxiliary for the asymmetric synthesis since 1983. Hisashi Yamamoto first utilized (''R'')-BINOL as a chiral auxiliary in the asymmetric synthesis oflimonene
Limonene () is a colorless liquid aliphatic hydrocarbon classified as a cyclic monoterpene, and is the major component in the essential oil of citrus fruit peels. The (+)-isomer, occurring more commonly in nature as the fragrance of oranges, ...
, which is an example of cyclic mono-terpene
Terpenes () are a class of natural products consisting of compounds with the formula (C5H8)n for n ≥ 2. Terpenes are major biosynthetic building blocks. Comprising more than 30,000 compounds, these unsaturated hydrocarbons are produced predomi ...
s. (''R'')-BINOL mononeryl ether was prepared by the monosilylation and alkylation of (''R'')-BINOL as the chiral auxiliary. Followed with the reduction by organoaluminum reagent, limonene was synthesized with low yields (29% yield) and moderate enantiomeric excesses up to 64% ee.
 The preparation of a variety of enantiomerically pure uncommon ''R''-amino acids can be achieved by the alkylation of chiral glycine derivatives possessing axially chiral BINOL as an auxiliary. It has been depicted by Fuji et al. Based on different
The preparation of a variety of enantiomerically pure uncommon ''R''-amino acids can be achieved by the alkylation of chiral glycine derivatives possessing axially chiral BINOL as an auxiliary. It has been depicted by Fuji et al. Based on different electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
, the diastereomeric excess varied from 69% to 86.
 Protected at the
Protected at the aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
function with (R)-BINOL, reacted diastereoselectively with Grignard reagents to afford protected atrolactaldehyde with moderate to excellent diastereomeric excess and high yields.
 BINOL was also used as a chiral auxiliary to control the formation of a P-stereocenter in an asymmetric metal-catalyzed C-P coupling process. Mondal et al. discovered that the Pd-catalysed C-P cross-coupling reaction between axially chiral BINOL-based phosphoramidites and aryl halides or triflates proceeds with excellent stereoselectivity due to the presence of BINOL near the reacting P center.
BINOL was also used as a chiral auxiliary to control the formation of a P-stereocenter in an asymmetric metal-catalyzed C-P coupling process. Mondal et al. discovered that the Pd-catalysed C-P cross-coupling reaction between axially chiral BINOL-based phosphoramidites and aryl halides or triflates proceeds with excellent stereoselectivity due to the presence of BINOL near the reacting P center.
''trans''-2-Phenylcyclohexanol
ester
In chemistry, an ester is a compound derived from an acid (either organic or inorganic) in which the hydrogen atom (H) of at least one acidic hydroxyl group () of that acid is replaced by an organyl group (R). These compounds contain a distin ...
of glyoxylic acid
Glyoxylic acid or oxoacetic acid is an organic compound. Together with acetic acid, glycolic acid, and oxalic acid, glyoxylic acid is one of the C2 carboxylic acids. It is a colourless solid that occurs naturally and is useful industrially.
Str ...
.
 In the total synthesis of (−)-heptemerone B and (−)-guanacastepene E, attached with trans-2-phenylcyclohexanol, the glyoxylate reacted with 2,4-dimethyl-pent-2-ene, in the presence of
In the total synthesis of (−)-heptemerone B and (−)-guanacastepene E, attached with trans-2-phenylcyclohexanol, the glyoxylate reacted with 2,4-dimethyl-pent-2-ene, in the presence of tin(IV) chloride
Tin(IV) chloride, also known as tin tetrachloride or stannic chloride, is an inorganic compound of tin and chlorine with the formula SnCl4. It is a colorless hygroscopic liquid, which fumes on contact with air. It is used as a precursor to other ...
, yielding the desired anti adduct as the major product, together with a small amount of its syn isomer with 10:1 diastereomeric ratio.
 For even greater conformational control, switching from a
For even greater conformational control, switching from a phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ...
to a trityl group gives ''trans''-2-tritylcyclohexanol (TTC). In 2015, the Brown group published an efficient chiral permanganate
A permanganate () is a chemical compound with the manganate(VII) ion, , the conjugate base of permanganic acid. Because the manganese atom has a +7 oxidation state, the permanganate(VII) ion is a strong oxidising agent. The ion is a transition ...
-mediated oxidative cyclization with TTC.

Oxazolidinones
Oxazolidinone auxiliaries, popularized by David A. Evans, have been applied to many stereoselective transformations, including aldol reactions,alkylation Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
reactions, and Diels-Alder reactions. The oxazolidinones are substituted at the 4 and 5 positions. Through steric hindrance, the substituents direct the direction of substitution of various groups. The auxiliary is subsequently removed e.g. through hydrolysis.
Preparation
Oxazolidinones can be prepared fromamino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s or readily available amino alcohols
In organic chemistry, alkanolamines (amino alcohols) are organic compounds that contain both hydroxyl () and amino (, , and ) functional groups on an alkane backbone. Alkanolamine's bifunctionality and physicochemical characteristics lead to its u ...
. A large number of oxazolidinones are commercially available, including the four below.
acyl chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
.

Alkylation reactions
Deprotonation at theα-carbon
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Numeric locants
The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of n ...
of an oxazolidinone imide
In organic chemistry, an imide is a functional group consisting of two acyl groups bound to nitrogen. The compounds are structurally related to acid anhydrides, although imides are more resistant to hydrolysis. In terms of commercial applications ...
with a strong base such as lithium diisopropylamide selectively furnishes the (''Z'')-enol
In organic chemistry, enols are a type of functional group or intermediate in organic chemistry containing a group with the formula (R = many substituents). The term ''enol'' is an abbreviation of ''alkenol'', a portmanteau deriving from "-ene ...
ate, which can undergo stereoselective alkylation Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
.
 Activated electrophiles, such as
Activated electrophiles, such as allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
ic or benzyl
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group ().
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substituent ...
ic halide
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fl ...
s, are very good substrates.
Aldol reactions
Chiral oxazolidinones have been employed most widely in stereoselective aldol reactions. Soft enolization with theLewis acid
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any ...
dibutylboron triflate and the base diisopropylethylamine gives the (''Z'')-enolate, which undergoes a diastereoselective aldol reaction with an aldehyde substrate. The transformation is particularly powerful because it establishes two contiguous stereocenters simultaneously.
 A model for the observed stereoselectivity can be found below. The ''syn''-stereo relationship between the methyl group and the new secondary alcohol results from a six-membered ring Zimmerman-Traxler
A model for the observed stereoselectivity can be found below. The ''syn''-stereo relationship between the methyl group and the new secondary alcohol results from a six-membered ring Zimmerman-Traxler transition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked w ...
, wherein the enolate oxygen and the aldehyde oxygen both coordinate boron. The aldehyde is oriented such that the hydrogen is placed in a pseudo-axial orientation to minimize 1,3-diaxial interactions. The absolute stereochemistry of the two stereocenters is controlled by the chirality in the auxiliary. In the transition structure, the auxiliary carbonyl is oriented away from the enolate oxygen so as to minimize the net dipole of the molecule; one face of the enolate is blocked by the substituent on the chiral auxiliary.

Removal
A variety of transformations have been developed to facilitate removal of the oxazolidinone auxiliary to generate different synthetically usefulfunctional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s.

Camphorsultam
Camphorsultam, or Oppolzer's sultam, is a classic chiral auxiliary.oxazoline
Oxazoline is a five-membered heterocyclic organic compound with the formula . It is the parent of a family of compounds called oxazolines (emphasis on plural), which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the ...
ring asymmetrically. Comparing with oxazolidinone as the chiral auxiliary, camphorsultam had a significant (2''S'',3''R'')-selectivity.
 Camphorsultam also acts as a chiral auxiliary in Michael addition. Lithium base promoted stereoselective Michael addition of thiols to N-mcthacryloylcamphorsultam produced the corresponding addition products in high diastereoselectivity.
Camphorsultam also acts as a chiral auxiliary in Michael addition. Lithium base promoted stereoselective Michael addition of thiols to N-mcthacryloylcamphorsultam produced the corresponding addition products in high diastereoselectivity.
 Camphorsultam was used as a chiral auxiliary for the asymmetric
Camphorsultam was used as a chiral auxiliary for the asymmetric Claisen rearrangement
The Claisen rearrangement is a powerful carbon–carbon chemical bond, bond-forming chemical reaction discovered by Rainer Ludwig Claisen. The heating of an allyl Vinyl group, vinyl ether will initiate a Sigmatropic reaction, ,3sigmatropic r ...
. In the presence of butylated hydroxytoluene
Butylated hydroxytoluene (BHT), also known as dibutylhydroxytoluene, is a lipophilic organic compound, chemically a derivative of phenol, that is useful for its antioxidant properties. BHT is widely used to prevent free radical-mediated oxidation ...
(BHT) used as a radical scavenger, a toluene solution of the adduct between geraniol
Geraniol is a monoterpenoid and an alcohol. It is the primary component of citronella oil and is a primary component of rose oil and palmarosa oil. It is a colorless oil, although commercial samples can appear yellow. It has low solubility i ...
and camphorsultam was heated in a sealed tube at 140 °C, to provide mainly the (2''R'',3''S'')-isomer as the major rearrangement product in 72% yield, securing the two contiguous stereocenters including the quaternary carbon.

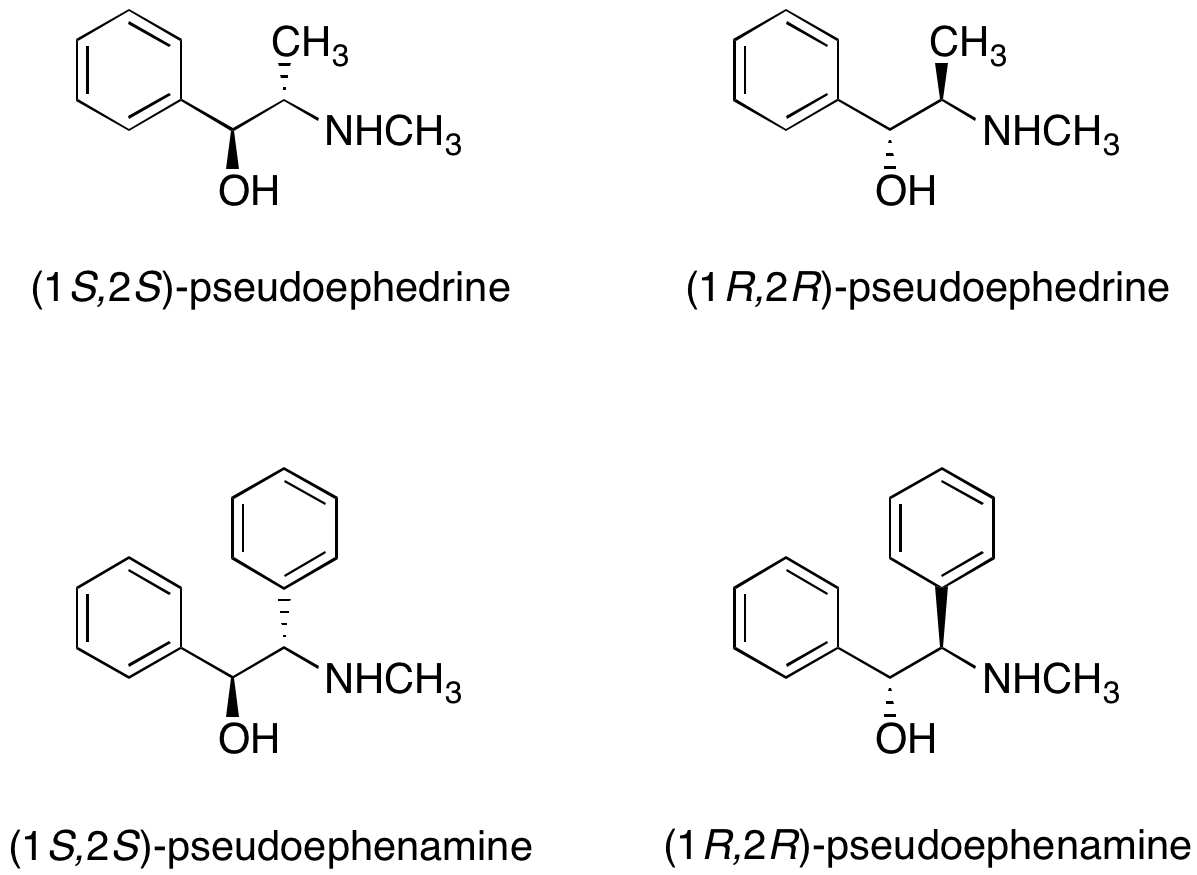
Pseudoephedrine and pseudoephenamine
Both (R,R)- and (S,S)-pseudoephedrine
Pseudoephedrine, sold under the brand name Sudafed among others, is a sympathomimetic medication which is used as a decongestant to treat nasal congestion. It has also been used off-label for certain other indications, like treatment of lo ...
can be used as chiral auxiliaries. Pseudoephedrine is reacted with a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
, acid anhydride
An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid.
In organic chemistry, organic acid anhydrides contain the functional group . Organic acid anhydrides often form when one equivalent of wa ...
, or acyl chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example o ...
to give the corresponding amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
.
The α-proton of the carbonyl compound is easily deprotonated by a non-nucleophilic base to give the enolate, which can further react. The configuration of the addition compound, such as with an alkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents of hydrogen atom. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalka ...
, is directed by the methyl group. Thus, any addition product will be syn with the methyl and anti to the hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
. The pseudoephedrine chiral auxiliary is subsequently removed by cleaving the amide bond with an appropriate nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
.
Preparation
Bothenantiomers
In chemistry, an enantiomer (Help:IPA/English, /ɪˈnænti.əmər, ɛ-, -oʊ-/ Help:Pronunciation respelling key, ''ih-NAN-tee-ə-mər''), also known as an optical isomer, antipode, or optical antipode, is one of a pair of molecular entities whi ...
of pseudoephedrine are commercially available. Racemic pseudoephedrine has many medical uses. Because pseudoephedrine can be used to illegally make methamphetamine
Methamphetamine (contracted from ) is a potent central nervous system (CNS) stimulant that is mainly used as a recreational drug use, recreational or Performance-enhancing substance, performance-enhancing drug and less commonly as a secon ...
, the purchase of pseudoephedrine for use in academic or industrial research is rather regulated. As an alternative, Myers et al. reported the utility of pseudoephenamine chiral auxiliaries in alkylation reactions. While pseudoephenamine is not readily available from commercial sources, it can be synthesized with relative ease from benzil and cannot be used to make amphetamine
Amphetamine (contracted from Alpha and beta carbon, alpha-methylphenethylamine, methylphenethylamine) is a central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, an ...
s.
 Pseudoephedrine amides are typically prepared by acylation with an acyl chloride or
Pseudoephedrine amides are typically prepared by acylation with an acyl chloride or anhydride
An acid anhydride is a type of chemical compound derived by the removal of water molecules from an acid (chemistry), acid.
In organic chemistry, organic acid anhydrides contain the functional group . Organic acid anhydrides often form when one ...
.

Alkylation
Pseudoephedrine amides undergo deprotonation by a strong base such as lithium diisopropylamide (LDA) to give the corresponding (''Z'')-enol
In organic chemistry, enols are a type of functional group or intermediate in organic chemistry containing a group with the formula (R = many substituents). The term ''enol'' is an abbreviation of ''alkenol'', a portmanteau deriving from "-ene ...
ates. Alkylation of these lithium enolates proceeds with high facial selectivity.

The diastereoselectivity is believed to result from a configuration wherein one face of the lithium enolate is blocked by the secondary lithium alkoxide and the solvent molecules associated with that lithium cation. In accordance with this proposal, it has been observed that the diastereoselectivity of the alkylation step is highly dependent on the amount of lithium chloride present and on the solvent,
tetrahydrofuran
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water- miscible organic liquid with low viscosity. It is ...
(THF). Typically, 4 to 6 equivalents of lithium chloride are sufficient to saturate a solution of enolate in THF at the reaction molarity.

One primary advantage of asymmetric alkylation with pseudoephedrine amides is that the amide enolates are typically nucleophilic enough to react with primary and even secondary halides at temperatures ranging from –78 °C to 0 °C. Construction of quaternary carbon centers by alkylation of α-branched amide enolates is also possible, though the addition of DMPU is necessary for less reactive electrophiles.
Removal
Conditions have been developed for the transformation of pseudoephedrine amides into enantiomerically enrichedcarboxylic acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
, alcohols
In chemistry, an alcohol (), is a type of organic compound that carries at least one hydroxyl () functional group bound to a Saturated and unsaturated compounds, saturated carbon atom. Alcohols range from the simple, like methanol and ethanol ...
, aldehydes
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
, and ketones
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
- after cleavage, the auxiliary can be recovered and reused.

''tert''-Butanesulfinamide
This specific sulfinamide chiral auxiliary was initially developed by Jonathan A. Ellman, and its use has been explored extensively by his group. Thus, it is often referred to as Ellman's auxiliary or Ellman's sulfinamide.
Preparation
Either enantiomer of ''tert''-butanesulfinamide can be reached from ''tert''-butyl disulfide in two steps: a catalytic asymmetric oxidation reaction gives the disulfide oxidation product (thiosulfinate) in high yield and enantiomeric excess. Treatment of this compound with lithium amide in ammonia affords optically pure inverted product.
Condensation of ''tert''-butanesulfinamide with an aldehyde or ketone proceeds in high yield and affords only the (''E'')-isomer of the corresponding ''N''-sulfinyl imines.

Synthesis of chiral amines
Addition of a Grignard reagent to a ''tert''-butanesulfinyl aldimine or ketimine results in asymmetric addition to give the branched sulfinamide. The observed stereoselectivity can be rationalized by a six-membered ring transition structure, wherein both oxygen and nitrogen of the sulfinyl imine coordinate magnesium.
Removal
The auxiliary can be removed from the desired amine by treatment withhydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
in protic solvent
In chemistry, a protic solvent is a solvent that has a hydrogen atom bound to an oxygen (as in a hydroxyl group ), a nitrogen (as in an amine group or ), or fluoride (as in hydrogen fluoride). In general terms, any solvent that contains a labi ...
s.

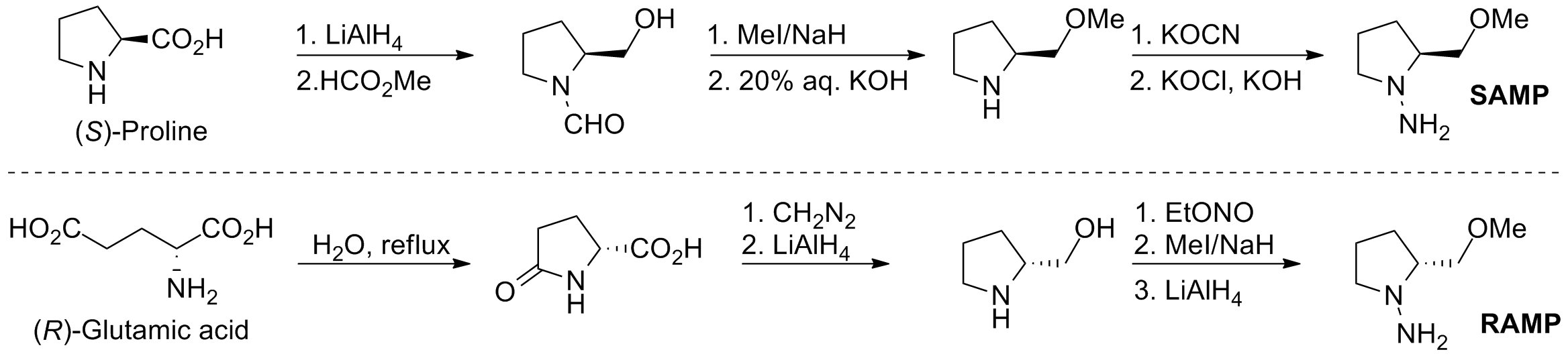
SAMP/RAMP
Alkylation reactions of chiral (''S'')-1-amino-2-methoxymethylpyrrolidine ( SAMP) and (''R'')-1-amino-2-methoxymethylpyrrolidine ( RAMP) hydrazones were developed by Dieter Enders and E.J. Corey.Preparation
SAMP can be prepared in six steps from (''S'')-proline, and RAMP can be prepared in six steps from (''R'')-glutamic acid.
Alkylation reactions
Condensation of SAMP or RAMP with an aldehyde or ketone affords the (''E'')-hydrazine. Deprotonation with lithium diisopropylamide and addition of an alkyl halide affords the alkylated product. The auxiliary can be removed byozonolysis
In organic chemistry, ozonolysis is an organic reaction where the Saturated and unsaturated compounds, unsaturated bonds are Bond cleavage, cleaved with ozone (). Multiple carbon–carbon bond are replaced by carbonyl () groups, such as aldehydes ...
or hydrolysis.

Chiral auxiliaries in industry
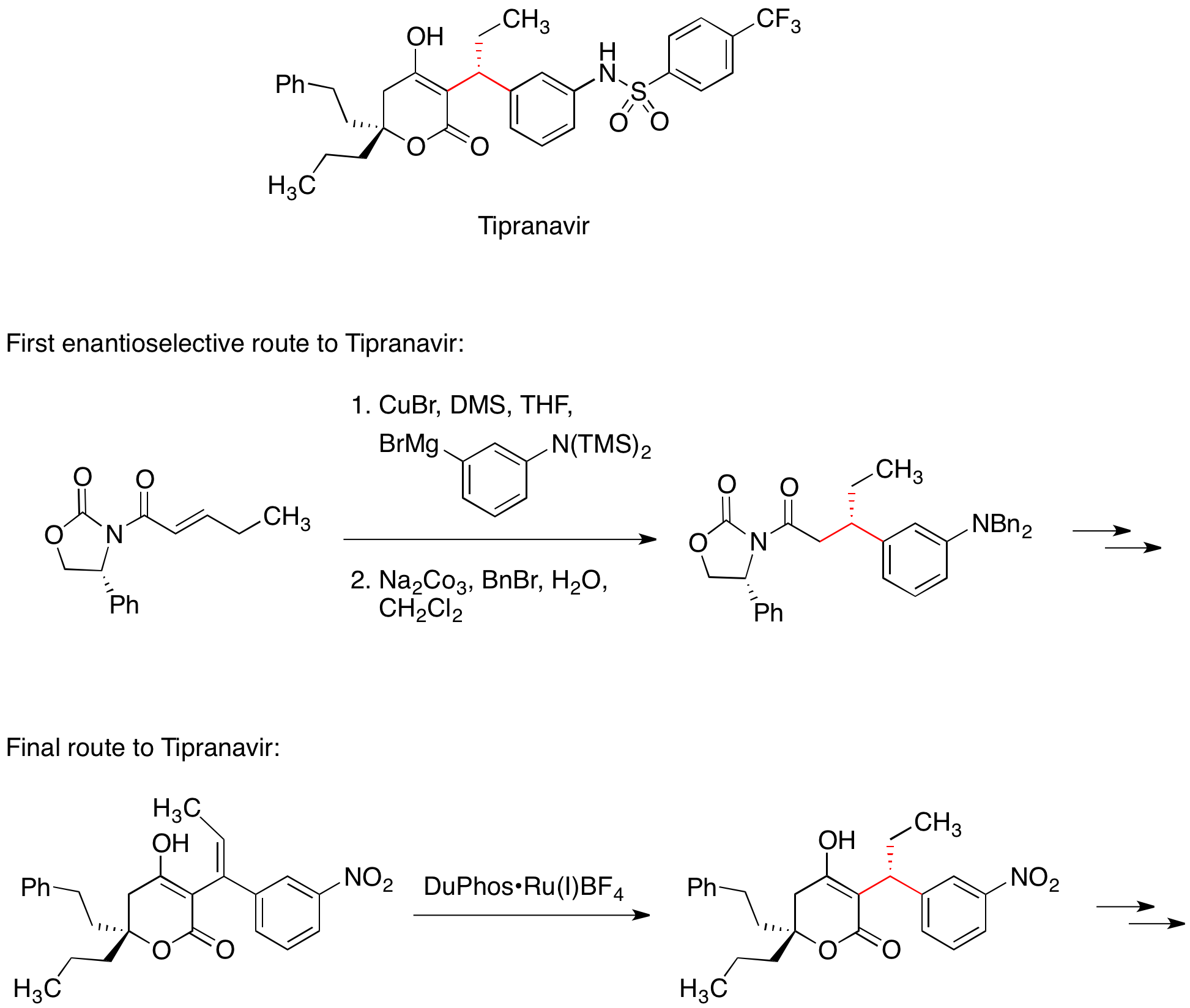
Chiral auxiliaries are generally reliable and versatile, enabling the synthesis of a large number of enantiomerically pure compounds in a time-efficient manner. Consequently, chiral auxiliaries are often the method of choice in the early phases of drug development.Tipranavir
The HIV protease inhibitor Tipranavir is marketed for the treatment of AIDS. The first enantioselective medicinal chemistry route to Tipranavir included the conjugate addition of an organocuprate reagent to a chiral Michael acceptor. The chiral oxazolidinone in the Michael acceptor controlled the stereochemistry of one of two stereocenters in the molecule. The final, commercial route to Tipranavir does not feature a chiral auxiliary; instead, this stereocenter is set by an asymmetric hydrogenation reaction.
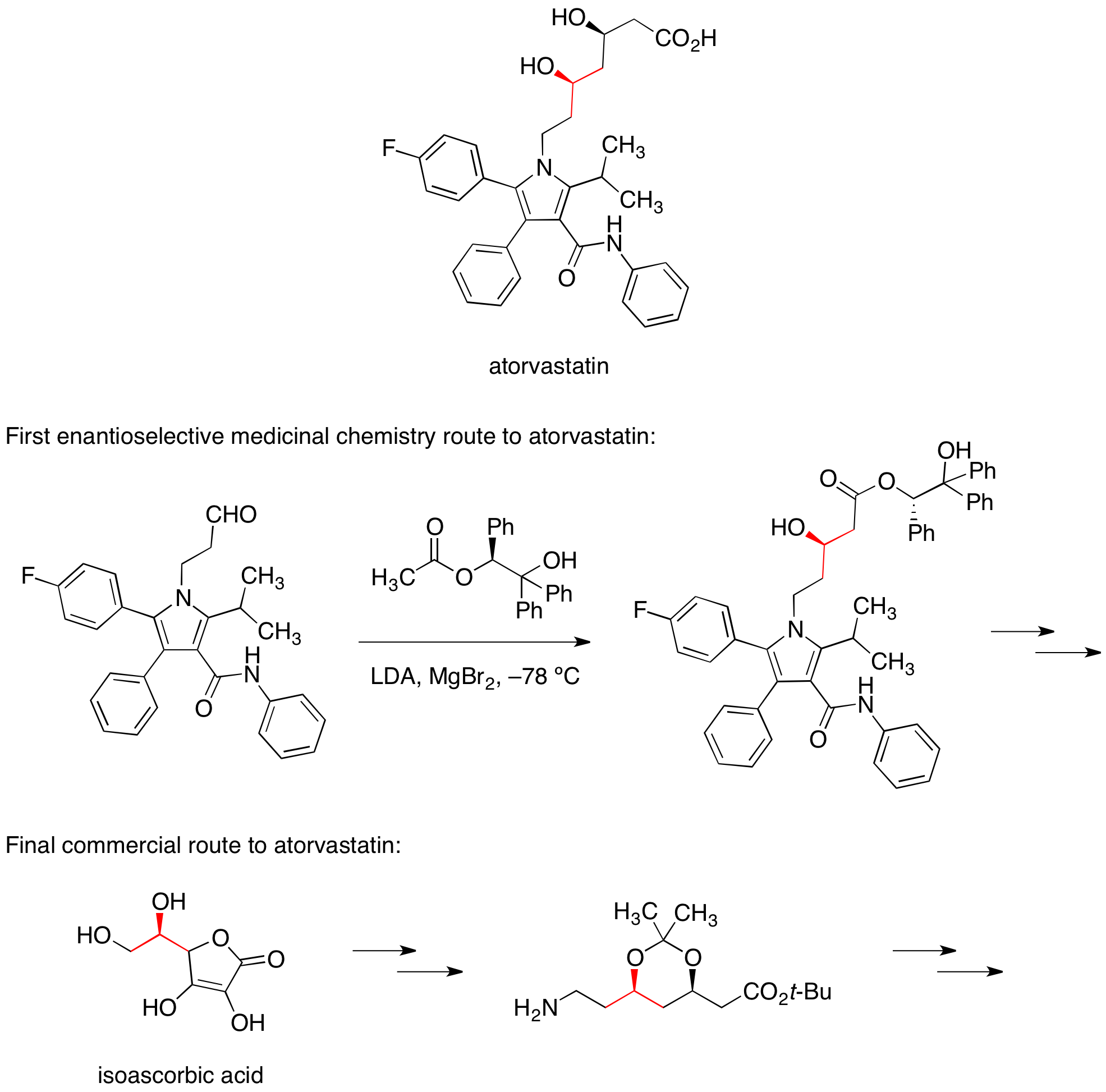
Atorvastatin
The calcium salt ofatorvastatin
Atorvastatin, sold under the brand name Lipitor among others, is a statin medication used to prevent cardiovascular disease in those at high risk and to treat abnormal lipid levels. For the prevention of cardiovascular disease, statins are a ...
is marketed under the trade name Lipitor for the lowering of blood cholesterol. The first enantioselective medicinal chemistry route to atorvastatin relied on a diastereoselective aldol reaction with a chiral ester to set one of the two alcohol stereocenters. In the commercial route to atorvastatin, this stereocenter is carried forward from the readily available food additive
Food additives are substances added to food to preserve flavor or enhance taste, appearance, or other sensory qualities. Some additives, such as vinegar ( pickling), salt ( salting), smoke ( smoking) and sugar ( crystallization), have been used f ...
isoascorbic acid.

See also
* Example of use of trans-2-phenyl-1-cyclohexanol as chiral auxiliary: Ojima lactam * Valine as a Chiral auxiliary in the Schöllkopf methodReferences
{{Chiral synthesis Stereochemistry