Chemiosmotic potential on:
[Wikipedia]
[Google]
[Amazon]
 An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a
An electrochemical gradient is a gradient of electrochemical potential, usually for an ion that can move across a
 Since the ions are charged, they cannot pass through cellular membranes via simple diffusion. Two different mechanisms can transport the ions across the membrane: active or passive transport.
An example of active transport of ions is the Na+-K+-ATPase (NKA). NKA is powered by the
Since the ions are charged, they cannot pass through cellular membranes via simple diffusion. Two different mechanisms can transport the ions across the membrane: active or passive transport.
An example of active transport of ions is the Na+-K+-ATPase (NKA). NKA is powered by the
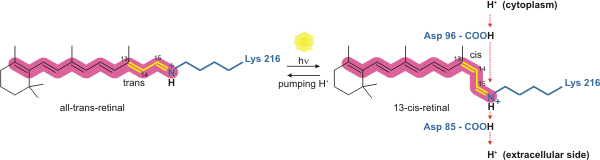 The way bacteriorhodopsin generates a proton gradient in
The way bacteriorhodopsin generates a proton gradient in
 PSII also relies on
PSII also relies on
4h\nu + 2H2O + 2PQ + 4H+ stroma -> O2 + 2PQH2 + 4H+ lumen
After being released from PSII, PQH2 travels to the cytochrome b6f complex, which then transfers two electrons from PQH2 to plastocyanin in two separate reactions. The process that occurs is similar to the Q-cycle in Complex III of the electron transport chain. In the first reaction, PQH2 binds to the complex on the lumen side and one electron is transferred to the iron-sulfur center which then transfers it to cytochrome f which then transfers it to plastocyanin. The second electron is transferred to heme bL which then transfers it to heme bH which then transfers it to PQ. In the second reaction, a second PQH2 gets oxidized, adding an electron to another plastocyanin and PQ. Both reactions together transfer four protons into the lumen.
 In the electron transport chain, complex I (CI) catalyzes the reduction of ubiquinone (UQ) to ubiquinol (UQH2) by the transfer of two
In the electron transport chain, complex I (CI) catalyzes the reduction of ubiquinone (UQ) to ubiquinol (UQH2) by the transfer of two
Stephen T. Abedon, "Important words and concepts from Chapter 8, Campbell & Reece, 2002 (1/14/2005)", for Biology 113 at the Ohio State University
{{DEFAULTSORT:Electrochemical Gradient Cellular respiration Electrochemical concepts Electrophysiology Membrane biology Physical quantities Thermodynamics
membrane
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Membranes can be generally classified into synthetic membranes and biological membranes. Bi ...
. The gradient consists of two parts:
* The chemical gradient, or difference in solute concentration across a membrane.
* The electrical gradient, or difference in charge across a membrane.
If there are unequal concentrations of an ion across a permeable membrane, the ion will move across the membrane from the area of higher concentration to the area of lower concentration through simple diffusion. Ions also carry an electric charge that forms an electric potential
Electric potential (also called the ''electric field potential'', potential drop, the electrostatic potential) is defined as electric potential energy per unit of electric charge. More precisely, electric potential is the amount of work (physic ...
across a membrane. If there is an unequal distribution of charges across the membrane, then the difference in electric potential generates a force that drives ion diffusion until the charges are balanced on both sides of the membrane.
Electrochemical gradients are essential to the operation of batteries and other electrochemical cell
An electrochemical cell is a device that either generates electrical energy from chemical reactions in a so called galvanic cell, galvanic or voltaic cell, or induces chemical reactions (electrolysis) by applying external electrical energy in an ...
s, photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
and cellular respiration
Cellular respiration is the process of oxidizing biological fuels using an inorganic electron acceptor, such as oxygen, to drive production of adenosine triphosphate (ATP), which stores chemical energy in a biologically accessible form. Cell ...
, and certain other biological process
Biological processes are those processes that are necessary for an organism to live and that shape its capacities for interacting with its environment. Biological processes are made of many chemical reactions or other events that are involved in ...
es.
Overview
Electrochemical energy is one of the many interchangeable forms ofpotential energy
In physics, potential energy is the energy of an object or system due to the body's position relative to other objects, or the configuration of its particles. The energy is equal to the work done against any restoring forces, such as gravity ...
through which energy may be conserved. It appears in electroanalytical chemistry and has industrial applications such as batteries and fuel cells. In biology, electrochemical gradients allow cells to control the direction ions move across membranes. In mitochondria
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is us ...
and chloroplast
A chloroplast () is a type of membrane-bound organelle, organelle known as a plastid that conducts photosynthesis mostly in plant cell, plant and algae, algal cells. Chloroplasts have a high concentration of chlorophyll pigments which captur ...
s, proton gradients generate a chemiosmotic potential used to synthesize ATP, and the sodium-potassium gradient helps neural synapses quickly transmit information.
An electrochemical gradient has two components: a differential concentration of electric charge
Electric charge (symbol ''q'', sometimes ''Q'') is a physical property of matter that causes it to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative''. Like charges repel each other and ...
across a membrane and a differential concentration of chemical species across that same membrane. In the former effect, the concentrated charge attracts charges of the opposite sign; in the latter, the concentrated species tends to diffuse across the membrane to an equalize concentrations. The combination of these two phenomena determines the thermodynamically-preferred direction for an ion's movement across the membrane.
The combined effect can be quantified as a gradient in the thermodynamic
Thermodynamics is a branch of physics that deals with heat, work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed by the four laws of th ...
electrochemical potential:
with
* the chemical potential of the ion species
* the charge per ion of the species
* , Faraday constant
In physical chemistry, the Faraday constant (symbol , sometimes stylized as ℱ) is a physical constant defined as the quotient of the total electric charge () by the amount () of elementary charge carriers in any given sample of matter: it ...
(the electrochemical potential is implicitly measured on a per- mole basis)
* , the local electric potential
Electric potential (also called the ''electric field potential'', potential drop, the electrostatic potential) is defined as electric potential energy per unit of electric charge. More precisely, electric potential is the amount of work (physic ...
.
Sometimes, the term "electrochemical potential" is abused to describe the electric potential ''generated'' by an ionic concentration gradient; that is, .
An electrochemical gradient is analogous to the water pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country and eve ...
across a hydroelectric dam. Routes unblocked by the membrane (e.g. membrane transport protein
A membrane transport protein is a membrane protein involved in the movement of ions, small molecules, and macromolecules, such as another protein, across a biological membrane. Transport proteins are integral membrane proteins, integral transmembr ...
or electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a varie ...
s) correspond to turbines that convert the water's potential energy to other forms of physical or chemical energy, and the ions that pass through the membrane correspond to water traveling into the lower river. Conversely, energy can be used to pump water up into the lake above the dam, and chemical energy can be used to create electrochemical gradients.
Chemistry
The term typically applies inelectrochemistry
Electrochemistry is the branch of physical chemistry concerned with the relationship between Electric potential, electrical potential difference and identifiable chemical change. These reactions involve Electron, electrons moving via an electronic ...
, when electrical energy
Electrical energy is the energy transferred as electric charges move between points with different electric potential, that is, as they move across a voltage, potential difference. As electric potential is lost or gained, work is done changing the ...
in the form of an applied voltage is used to modulate the thermodynamic favorability of a chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
. In a battery, an electrochemical potential arising from the movement of ions balances the reaction energy of the electrodes. The maximum voltage that a battery reaction can produce is sometimes called the standard electrochemical potential of that reaction.
Biological context
The generation of a transmembrane electrical potential through ion movement across acell membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
drives biological process
Biological processes are those processes that are necessary for an organism to live and that shape its capacities for interacting with its environment. Biological processes are made of many chemical reactions or other events that are involved in ...
es like nerve
A nerve is an enclosed, cable-like bundle of nerve fibers (called axons). Nerves have historically been considered the basic units of the peripheral nervous system. A nerve provides a common pathway for the Electrochemistry, electrochemical nerv ...
conduction, muscle contraction
Muscle contraction is the activation of Tension (physics), tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in musc ...
, hormone
A hormone (from the Ancient Greek, Greek participle , "setting in motion") is a class of cell signaling, signaling molecules in multicellular organisms that are sent to distant organs or tissues by complex biological processes to regulate physio ...
secretion
Secretion is the movement of material from one point to another, such as a secreted chemical substance from a cell or gland. In contrast, excretion is the removal of certain substances or waste products from a cell or organism. The classical mec ...
, and sensation. By convention, physiological voltages are measured relative to the extracellular region; a typical animal cell has an internal electrical potential of (−70)–(−50) mV.
An electrochemical gradient is essential to mitochondrial oxidative phosphorylation
Oxidative phosphorylation(UK , US : or electron transport-linked phosphorylation or terminal oxidation, is the metabolic pathway in which Cell (biology), cells use enzymes to Redox, oxidize nutrients, thereby releasing chemical energy in order ...
. The final step of cellular respiration
Cellular respiration is the process of oxidizing biological fuels using an inorganic electron acceptor, such as oxygen, to drive production of adenosine triphosphate (ATP), which stores chemical energy in a biologically accessible form. Cell ...
is the electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
, composed of four complexes embedded in the inner mitochondrial membrane. Complexes I, III, and IV pump protons from the matrix
Matrix (: matrices or matrixes) or MATRIX may refer to:
Science and mathematics
* Matrix (mathematics), a rectangular array of numbers, symbols or expressions
* Matrix (logic), part of a formula in prenex normal form
* Matrix (biology), the m ...
to the intermembrane space
The intermembrane space (IMS) is the space occurring between or involving two or more membranes. In cell biology, it is most commonly described as the region between the Inner mitochondrial membrane, inner membrane and the Outer mitochondrial memb ...
(IMS); for every electron pair entering the chain, ten protons translocate into the IMS. The result is an electric potential of more than . The energy resulting from the flux of protons back into the matrix is used by ATP synthase to combine inorganic phosphate
Phosphates are the naturally occurring form of the element phosphorus.
In chemistry, a phosphate is an anion, salt, functional group or ester derived from a phosphoric acid. It most commonly means orthophosphate, a derivative of orthop ...
and ADP.
Similar to the electron transport chain, the light-dependent reactions of photosynthesis pump protons into the thylakoid
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacterium, cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a #Membrane, thylakoid membrane surrounding a #Lumen, ...
lumen of chloroplasts to drive the synthesis of ATP. The proton gradient can be generated through either noncyclic or cyclic photophosphorylation. Of the proteins that participate in noncyclic photophosphorylation, photosystem II (PSII), plastiquinone, and cytochrome b6f complex directly contribute to generating the proton gradient. For each four photons absorbed by PSII, eight protons are pumped into the lumen.
Several other transporters and ion channels play a role in generating a proton electrochemical gradient. One is TPK3, a potassium channel that is activated by Ca2+ and conducts K+ from the thylakoid lumen to the stroma, which helps establish the electric field
An electric field (sometimes called E-field) is a field (physics), physical field that surrounds electrically charged particles such as electrons. In classical electromagnetism, the electric field of a single charge (or group of charges) descri ...
. On the other hand, the electro-neutral K+ efflux antiporter
An antiporter (also called exchanger or counter-transporter) is an integral membrane protein that uses secondary active transport to move two or more molecules in opposite directions across a phospholipid membrane. It is a type of cotransporte ...
(KEA3) transports K+ into the thylakoid lumen and H+ into the stroma, which helps establish the pH gradient.
Ion gradients
hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of ATP into ADP and an inorganic phosphate; for every molecule of ATP hydrolized, three Na+ are transported outside and two K+ are transported inside the cell. This makes the inside of the cell more negative than the outside and more specifically generates a membrane potential ''V''membrane of about .
An example of passive transport is ion fluxes through Na+, K+, Ca2+, and Cl− channels. Unlike active transport, passive transport is powered by the arithmetic sum of osmosis
Osmosis (, ) is the spontaneous net movement or diffusion of solvent molecules through a selectively permeable membrane, selectively-permeable membrane from a region of high water potential (region of lower solute concentration) to a region of ...
(a concentration gradient) and an electric field
An electric field (sometimes called E-field) is a field (physics), physical field that surrounds electrically charged particles such as electrons. In classical electromagnetism, the electric field of a single charge (or group of charges) descri ...
(the transmembrane potential). Formally, the molar Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
change associated with successful transport is where represents the gas constant, represents absolute temperature
Thermodynamic temperature, also known as absolute temperature, is a physical quantity which measures temperature starting from absolute zero, the point at which particles have minimal thermal motion.
Thermodynamic temperature is typically expres ...
, is the charge per ion, and represents the Faraday constant
In physical chemistry, the Faraday constant (symbol , sometimes stylized as ℱ) is a physical constant defined as the quotient of the total electric charge () by the amount () of elementary charge carriers in any given sample of matter: it ...
.
In the example of Na+, both terms tend to support transport: the negative electric potential inside the cell attracts the positive ion and since Na+ is concentrated outside the cell, osmosis supports diffusion through the Na+ channel into the cell. In the case of K+, the effect of osmosis is reversed: although external ions are attracted by the negative intracellular potential, entropy seeks to diffuse the ions already concentrated inside the cell. The converse phenomenon (osmosis supports transport, electric potential opposes it) can be achieved for Na+ in cells with abnormal transmembrane potentials: at , the Na+ influx halts; at higher potentials, it becomes an efflux.
Proton gradients
Proton gradients in particular are important in many types of cells as a form of energy storage. The gradient is usually used to drive ATP synthase, flagellar rotation, ormetabolite
In biochemistry, a metabolite is an intermediate or end product of metabolism.
The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, c ...
transport. This section will focus on three processes that help establish proton gradients in their respective cells: bacteriorhodopsin and noncyclic photophosphorylation and oxidative phosphorylation.
Bacteriorhodopsin
 The way bacteriorhodopsin generates a proton gradient in
The way bacteriorhodopsin generates a proton gradient in Archaea
Archaea ( ) is a Domain (biology), domain of organisms. Traditionally, Archaea only included its Prokaryote, prokaryotic members, but this has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even thou ...
is through a proton pump
A proton pump is an integral membrane protein pump that builds up a proton gradient across a biological membrane. Proton pumps catalyze the following reaction:
: n one side of a biological membrane/sub> + energy n the other side of the m ...
. The proton pump relies on proton carriers to drive protons from the side of the membrane with a low H+ concentration to the side of the membrane with a high H+ concentration. In bacteriorhodopsin, the proton pump is activated by absorption of photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
s of 568nm wavelength
In physics and mathematics, wavelength or spatial period of a wave or periodic function is the distance over which the wave's shape repeats.
In other words, it is the distance between consecutive corresponding points of the same ''phase (waves ...
, which leads to isomerization of the Schiff base (SB) in retinal
Retinal (also known as retinaldehyde) is a polyene chromophore. Retinal, bound to proteins called opsins, is the chemical basis of visual phototransduction, the light-detection stage of visual perception (vision).
Some microorganisms use ret ...
forming the K state. This moves SB away from Asp85 and Asp212, causing H+ transfer from the SB to Asp85 forming the M1 state. The protein then shifts to the M2 state by separating Glu204 from Glu194 which releases a proton from Glu204 into the external medium. The SB is reprotonated by Asp96 which forms the N state. It is important that the second proton comes from Asp96 since its deprotonated state is unstable and rapidly reprotonated with a proton from the cytosol
The cytosol, also known as cytoplasmic matrix or groundplasm, is one of the liquids found inside cells ( intracellular fluid (ICF)). It is separated into compartments by membranes. For example, the mitochondrial matrix separates the mitochondri ...
. The protonation of Asp85 and Asp96 causes re-isomerization of the SB, forming the O state. Finally, bacteriorhodopsin returns to its resting state when Asp85 releases its proton to Glu204.
Photophosphorylation
light
Light, visible light, or visible radiation is electromagnetic radiation that can be visual perception, perceived by the human eye. Visible light spans the visible spectrum and is usually defined as having wavelengths in the range of 400– ...
to drive the formation of proton gradients in chloroplasts, however, PSII utilizes vectorial redox chemistry to achieve this goal. Rather than physically transporting protons through the protein, reactions requiring the binding of protons will occur on the extracellular side while reactions requiring the release of protons will occur on the intracellular side. Absorption of photons of 680nm wavelength is used to excite two electrons in P680 to a higher energy level
A quantum mechanics, quantum mechanical system or particle that is bound state, bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical mechanics, classical pa ...
. These higher energy electrons are transferred to protein-bound plastoquinone (PQA) and then to unbound plastoquinone (PQB). This reduces plastoquinone (PQ) to plastoquinol (PQH2) which is released from PSII after gaining two protons from the stroma. The electrons in P680 are replenished by oxidizing water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
through the oxygen-evolving complex (OEC). This results in release of O2 and H+ into the lumen, for a total reaction of
Oxidative phosphorylation
Main article:Oxidative phosphorylation
Oxidative phosphorylation(UK , US : or electron transport-linked phosphorylation or terminal oxidation, is the metabolic pathway in which Cell (biology), cells use enzymes to Redox, oxidize nutrients, thereby releasing chemical energy in order ...
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s from reduced nicotinamide adenine dinucleotide
Nicotinamide adenine dinucleotide (NAD) is a Cofactor (biochemistry), coenzyme central to metabolism. Found in all living cell (biology), cells, NAD is called a dinucleotide because it consists of two nucleotides joined through their phosphat ...
(NADH) which translocates four protons from the mitochondrial matrix to the IMS:
Complex III (CIII) catalyzes the Q-cycle. The first step involving the transfer of two electrons from the UQH2 reduced by CI to two molecules of oxidized cytochrome c at the Qo site. In the second step, two more electrons reduce UQ to UQH2 at the Qi site. The total reaction is:
Complex IV (CIV) catalyzes the transfer of two electrons from the cytochrome c reduced by CIII to one half of a full oxygen. Utilizing one full oxygen in oxidative phosphorylation requires the transfer of four electrons. The oxygen will then consume four protons from the matrix to form water while another four protons are pumped into the IMS, to give a total reaction
See also
* Concentration cell * Transmembrane potential difference *Action potential
An action potential (also known as a nerve impulse or "spike" when in a neuron) is a series of quick changes in voltage across a cell membrane. An action potential occurs when the membrane potential of a specific Cell (biology), cell rapidly ri ...
* Cell potential
* Electrodiffusion
* Galvanic cell
* Electrochemical cell
An electrochemical cell is a device that either generates electrical energy from chemical reactions in a so called galvanic cell, galvanic or voltaic cell, or induces chemical reactions (electrolysis) by applying external electrical energy in an ...
* Proton exchange membrane
* Reversal potential
References
*Stephen T. Abedon, "Important words and concepts from Chapter 8, Campbell & Reece, 2002 (1/14/2005)", for Biology 113 at the Ohio State University
{{DEFAULTSORT:Electrochemical Gradient Cellular respiration Electrochemical concepts Electrophysiology Membrane biology Physical quantities Thermodynamics