Carbonyl alpha-substitution reactions on:
[Wikipedia]
[Google]
[Amazon]
Alpha-substitution reactions occur at the position next to the carbonyl group,
the α-position, and involve the 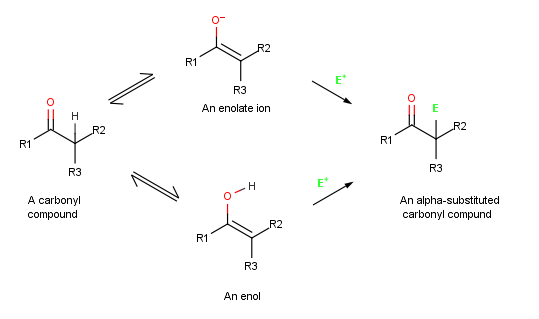
 When an alkene reacts with an electrophile, such as
When an alkene reacts with an electrophile, such as
 Alkylation reactions are subject to the same constraints that affect all SN2 reactions. Thus, the leaving group X in the alkylating agent R-X can be
Alkylation reactions are subject to the same constraints that affect all SN2 reactions. Thus, the leaving group X in the alkylating agent R-X can be
substitution
Substitution may refer to:
Arts and media
*Chord substitution, in music, swapping one chord for a related one within a chord progression
*Substitution (poetry), a variation in poetic scansion
* "Substitution" (song), a 2009 song by Silversun Pic ...
of an α hydrogen
Hydrogen is the chemical element with the symbol H and atomic number 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of diatomic molecules having the formula . It is colorless, odorless, tasteless, non-toxic ...
atom
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas ...
by an electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that ca ...
, E, through either an enol
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (). The te ...
or enolate ion
In organic chemistry, alkenols (shortened to enols) are a type of reactive structure or intermediate in organic chemistry that is represented as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond (). The ter ...
intermediate.

Reaction mechanism
Because their double bonds are electron rich, enols behave asnucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
s and react with electrophiles in much the same way that alkenes
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
do. But because of resonance electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary partic ...
donation of a lonepair of electrons on the neighboring oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
, enols are more electron- rich and correspondingly more reactive than alkenes. Notice in the following electrostatic potential map of ethenol (H2C=CHOH) how there is a substantial amount of electron density on the α carbon.
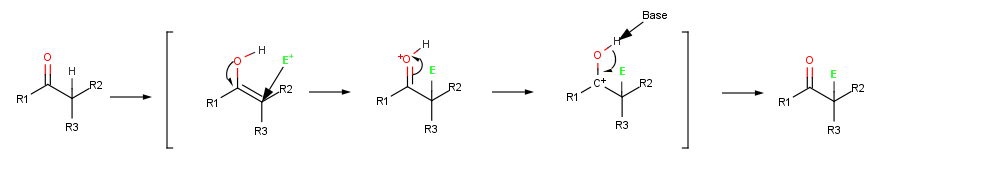
 When an alkene reacts with an electrophile, such as
When an alkene reacts with an electrophile, such as HCl HCL may refer to:
Science and medicine
* Hairy cell leukemia, an uncommon and slowly progressing B cell leukemia
* Harvard Cyclotron Laboratory, from 1961 to 2002, a proton accelerator used for research and development
* Hollow-cathode lamp, a s ...
, initial addition
Addition (usually signified by the plus symbol ) is one of the four basic operations of arithmetic, the other three being subtraction, multiplication and division. The addition of two whole numbers results in the total amount or ''sum'' of ...
of H+ gives an intermediate cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
and subsequent reaction with Cl− yields an addition product
Product may refer to:
Business
* Product (business), an item that serves as a solution to a specific consumer problem.
* Product (project management), a deliverable or set of deliverables that contribute to a business solution
Mathematics
* Prod ...
. When an enol reacts with an electrophile, however, only the initial addition step is the same. Instead of reacting with CI− to give an addition product, the intermediate cation loses the OH− proton to give an α-substituted carbonyl compound
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containing a ...
.
Alpha-halogenation of aldehydes and ketones
A particularly common α-substitution reaction in thelaboratory
A laboratory (; ; colloquially lab) is a facility that provides controlled conditions in which scientific or technological research, experiments, and measurement may be performed. Laboratory services are provided in a variety of settings: physici ...
is the halogenation
In chemistry, halogenation is a chemical reaction that entails the introduction of one or more halogens into a compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polyme ...
of aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
and ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
at their α positions by reaction Cl2, Br2 or I2 in acidic solution. Bromine
Bromine is a chemical element with the symbol Br and atomic number 35. It is the third-lightest element in group 17 of the periodic table (halogens) and is a volatile red-brown liquid at room temperature that evaporates readily to form a simil ...
in acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main componen ...
solvent is often used.
Remarkably, ketone halogenation also occurs in biological systems
A biological system is a complex network which connects several biologically relevant entities. Biological organization spans several scales and are determined based different structures depending on what the system is. Examples of biological syst ...
, particularly in marine alga, where dibromoacetaldehyde, bromoacetone
Bromoacetone is an organic compound with the formula . It is a colorless liquid although impure samples appear yellow or even brown. It is a lachrymatory agent and a precursor to other organic compounds.
Occurrence in nature
Bromoacetone is pr ...
, 1, l,l -tribromoacetone, and other related compounds have been found.
The halogenation is a typical α-substitution reaction that proceeds by acid catalyzed formation of an enol intermediate.
Acidity of alpha-hydrogen atoms: enolate ion formation
A hydrogen on the α position of a carbonyl compound is weaklyacidic
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a s ...
and can be removed by a strong base to yield an enolate ion. In comparing acetone
Acetone (2-propanone or dimethyl ketone), is an organic compound with the formula . It is the simplest and smallest ketone (). It is a colorless, highly volatile and flammable liquid with a characteristic pungent odour.
Acetone is miscible wi ...
(pKa= 19.3) with ethane
Ethane ( , ) is an organic chemical compound with chemical formula . At standard temperature and pressure, ethane is a colorless, odorless gas. Like many hydrocarbons, ethane is isolated on an industrial scale from natural gas and as a petro ...
(pKa= 60), for instance, the presence of a neighboring carbonyl group increases the acidity of the ketone over the alkane by a factor of 1040.
Abstraction of a proton from a carbonyl compound occurs when the a C-H bond is oriented roughly parallel to the p orbital
Orbital may refer to:
Sciences Chemistry and physics
* Atomic orbital
* Molecular orbital
* Hybrid orbital Astronomy and space flight
* Orbit
** Earth orbit
Medicine and physiology
* Orbit (anatomy), also known as the ''orbital bone''
* Orbito ...
s of the carbonyl group. The α carbon atom of the enolate ion is sp2-hybridized and has a p orbital that overlaps the neighboring carbonyl p orbitals. Thus, the negative charge
Charge or charged may refer to:
Arts, entertainment, and media Films
* ''Charge, Zero Emissions/Maximum Speed'', a 2011 documentary
Music
* ''Charge'' (David Ford album)
* ''Charge'' (Machel Montano album)
* ''Charge!!'', an album by The Aqua ...
is shared by the electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
oxygen atom, and the enolate ion is stabilized by resonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillat ...
.
Carbonyl compounds are more acidic than alkanes for the same reason that carboxylic acids
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an R-group. The general formula of a carboxylic acid is or , with R referring to the alkyl, alkenyl, aryl, or other group. Carboxyl ...
are more acidic than alcohols. In both cases, the anions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
are stabilized by resonance. Enolate ions
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
differ from carboxylate ions, however, in that their two resonance forms are not equivalent- the form with the negative charge on oxygen is lower in energy than the form with the charge on carbon. Nevertheless, the principle behind resonance stabilization is the same in both cases.
Because carbonyl compounds are only weakly acidic, a strong base is needed for enolate ion formation . If an alkoxide such as sodium ethoxide is used as base, deprotonation takes place only to the extent of about 0.1% because acetone is a weaker acid than ethanol (pKa= 16). If, however, a more powerful base such as sodium hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in ...
(NaH) or lithium diisopropylamide ( LDA) is used, a carbonyl compound can be completely converted into its enolate ion. Lithium diisopropylamide (LDA), which is easily prepared by reaction of the strong base butyllithium Butyllithium may refer to one of 5 isomeric organolithium reagents of which 3 are commonly used in chemical synthesis:
* ''n''-Butyllithium, abbreviated BuLi or nBuLi
* ''sec''-Butyllithium, abbreviated ''sec''-BuLi or sBuLi, has 2 stereoisomers, ...
with diisopropylamine
Diisopropylamine is a secondary amine with the chemical formula (Me2CH)2NH (Me = methyl). Diisopropylamine is a colorless liquid with an ammonia-like odor. Its lithium derivative, lithium diisopropylamide, known as LDA is a widely used reagent.
...
, is widely used in the laboratory as a base for preparing enolate ions from carbonyl compounds.
Many types of carbonyl compounds, including aldehydes
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl grou ...
, ketones
In organic chemistry, a ketone is a functional group with the structure R–C(=O)–R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group –C(=O)– (which contains a carbon-oxygen double bon ...
, esters
In chemistry, an ester is a compound derived from an oxoacid (organic or inorganic) in which at least one hydroxyl group () is replaced by an alkoxy group (), as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are ...
, thioesters
In organic chemistry, thioesters are organosulfur compounds with the functional group . They are analogous to carboxylate esters () with the sulfur in the thioester playing the role of the linking oxygen in the carboxylate ester, as implied by t ...
, acids
In computer science, ACID ( atomicity, consistency, isolation, durability) is a set of properties of database transactions intended to guarantee data validity despite errors, power failures, and other mishaps. In the context of databases, a ...
, and amides
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a compound with the general formula , where R, R', and R″ represent organic groups or hydrogen atoms. The amide group is called a peptide bond when it i ...
, can be converted into enolate ions by reaction with LDA. Note that nitriles
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix '' cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including m ...
, too, are acidic and can be converted into enolate-like anions (referred to as nitrile anion Nitrile anions is jargon from the organic product resulting from the deprotonation of alkyl nitriles. The proton(s) α to the nitrile group are sufficiently acidic that they undergo deprotonation by strong bases, usually lithium-derived. The produ ...
s).
When a hydrogen atom is flanked by two carbonyl groups, its acidity is enhanced even more. This enhanced acidity of β-dicarbonyl compounds is due to the stabilization of the resultant enolate ions by delocalization of the negative charge over both carbonyl groups.
Reactivity of enolate ions
Enolate ions are more useful than enols for two reasons. First, pure enols can't normally be isolated but are instead generated only as short lived intermediates in lowconcentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'' ...
. By contrast, stable solutions of pure enolate ions
are easily prepared from most carbonyl compounds by reaction with a strong base. Second, enolate ions are more reactive than enols and undergo many reactions that enols don't. Whereas enols are neutral, enolate ions are negatively charged, making them much better nucleophiles. As a result, enolate ions are more common than enols in both laboratory and biological chemistry.
Because they are resonance hybrids of two nonequivalent forms, enolate ions can be looked at either as vinylic alkoxides
In chemistry, an alkoxide is the conjugate base of an alcohol and therefore consists of an organic group bonded to a negatively charged oxygen atom. They are written as , where R is the organic substituent. Alkoxides are strong bases and, wh ...
(C=C- O−) or as α-ketocarbanions (−C-C= O). Thus, enolate ions can react with electrophiles either on oxygen or on carbon. Reaction on oxygen yields an enol derivative, while reaction on carbon yields an α-substituted carbonyl compound. Both kinds of reactivity are known, but reaction on carbon is more common.
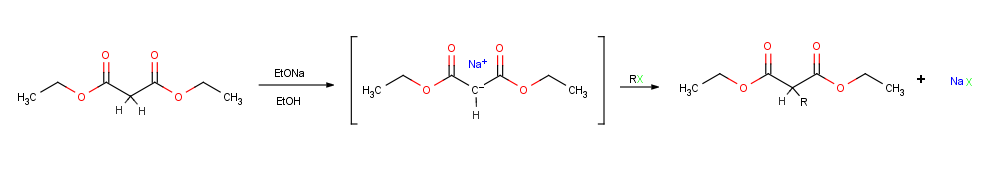
Alkylation of enolate ions
Perhaps the single most important reaction of enolate ions is their alkylation by treatment with analkyl halide
The haloalkanes (also known as halogenoalkanes or alkyl halides) are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely us ...
or tosylate
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula –. It consists of a tolyl group, –, joined to a sulfonyl group, ––, with the open valence on ...
, thereby forming a new C-C bond and joining two smaller pieces into one larger molecule
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bio ...
. Alkylation occurs when the nucleophilic enolate ion reacts with the electrophilic alkyl halide in an SN2 reaction and displaces the leaving group In chemistry, a leaving group is defined by the IUPAC as an atom or group of atoms that detaches from the main or residual part of a substrate during a reaction or elementary step of a reaction. However, in common usage, the term is often limited ...
by backside attack.
 Alkylation reactions are subject to the same constraints that affect all SN2 reactions. Thus, the leaving group X in the alkylating agent R-X can be
Alkylation reactions are subject to the same constraints that affect all SN2 reactions. Thus, the leaving group X in the alkylating agent R-X can be chloride
The chloride ion is the anion (negatively charged ion) Cl−. It is formed when the element chlorine (a halogen) gains an electron or when a compound such as hydrogen chloride is dissolved in water or other polar solvents. Chloride s ...
, bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retardan ...
, iodide
An iodide ion is the ion I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine def ...
, or tosylate
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or Tos) is a univalent functional group with the chemical formula –. It consists of a tolyl group, –, joined to a sulfonyl group, ––, with the open valence on ...
. Tile alkyl group R should be primary or methyl, and preferably should be allylic
In organic chemistry, an allyl group is a substituent with the structural formula , where R is the rest of the molecule. It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . ...
or benzylic
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure . Benzyl features a benzene ring () attached to a methylene group () group.
Nomenclature
In IUPAC nomenclature, the prefix benzyl refers to a substi ...
. Secondary halides react poorly, and tertiary halides don't react at all because a competing E2 elimination
Elimination may refer to:
Science and medicine
* Elimination reaction, an organic reaction in which two functional groups split to form an organic product
*Bodily waste elimination, discharging feces, urine, or foreign substances from the bo ...
of HX occurs instead. Vinylic and aryl halides are also unreactive because backside approach is sterically prevented.
References
{{reflist Substitution reactions Organic reactions