Carbonyl Allylation on:
[Wikipedia]
[Google]
[Amazon]
In 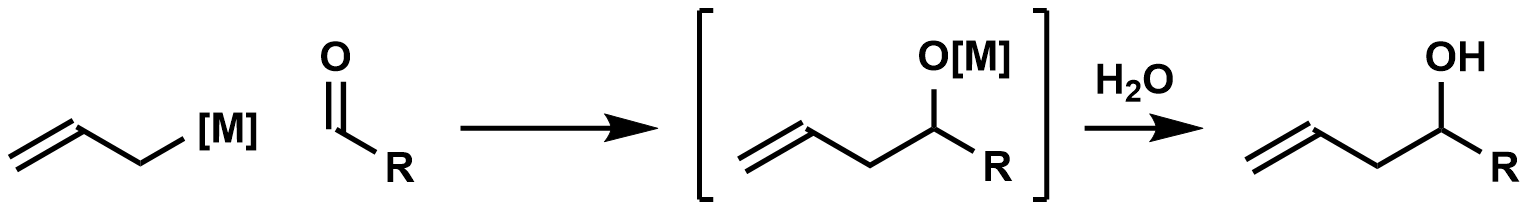
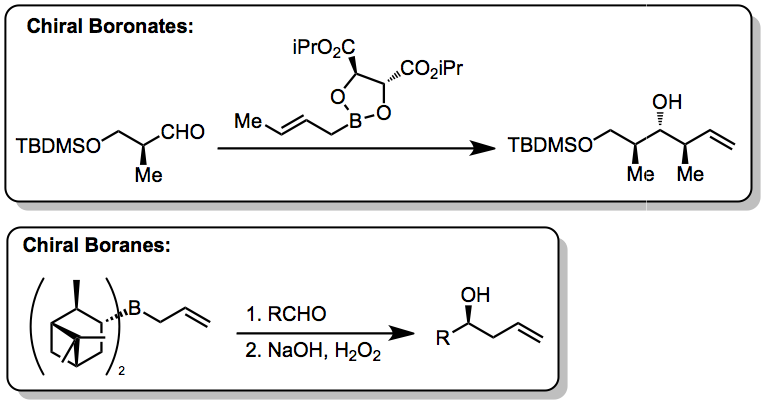 As illustrated by the Keck allylation, catalytic enantioselective additions of achiral allylmetal reagents to carbonyl compounds also are possible by
As illustrated by the Keck allylation, catalytic enantioselective additions of achiral allylmetal reagents to carbonyl compounds also are possible by 

organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
, carbonyl allylation describes methods for adding an allyl
In organic chemistry, an allyl group is a substituent with the structural formula . It consists of a methylene bridge () attached to a vinyl group (). The name is derived from the scientific name for garlic, . In 1844, Theodor Wertheim isolated a ...
anion to an aldehyde or ketone to produce a homoallylic alcohol. The carbonyl allylation was first reported in 1876 by Alexander Zaitsev and employed an allylzinc reagent.

Enantioselective versions
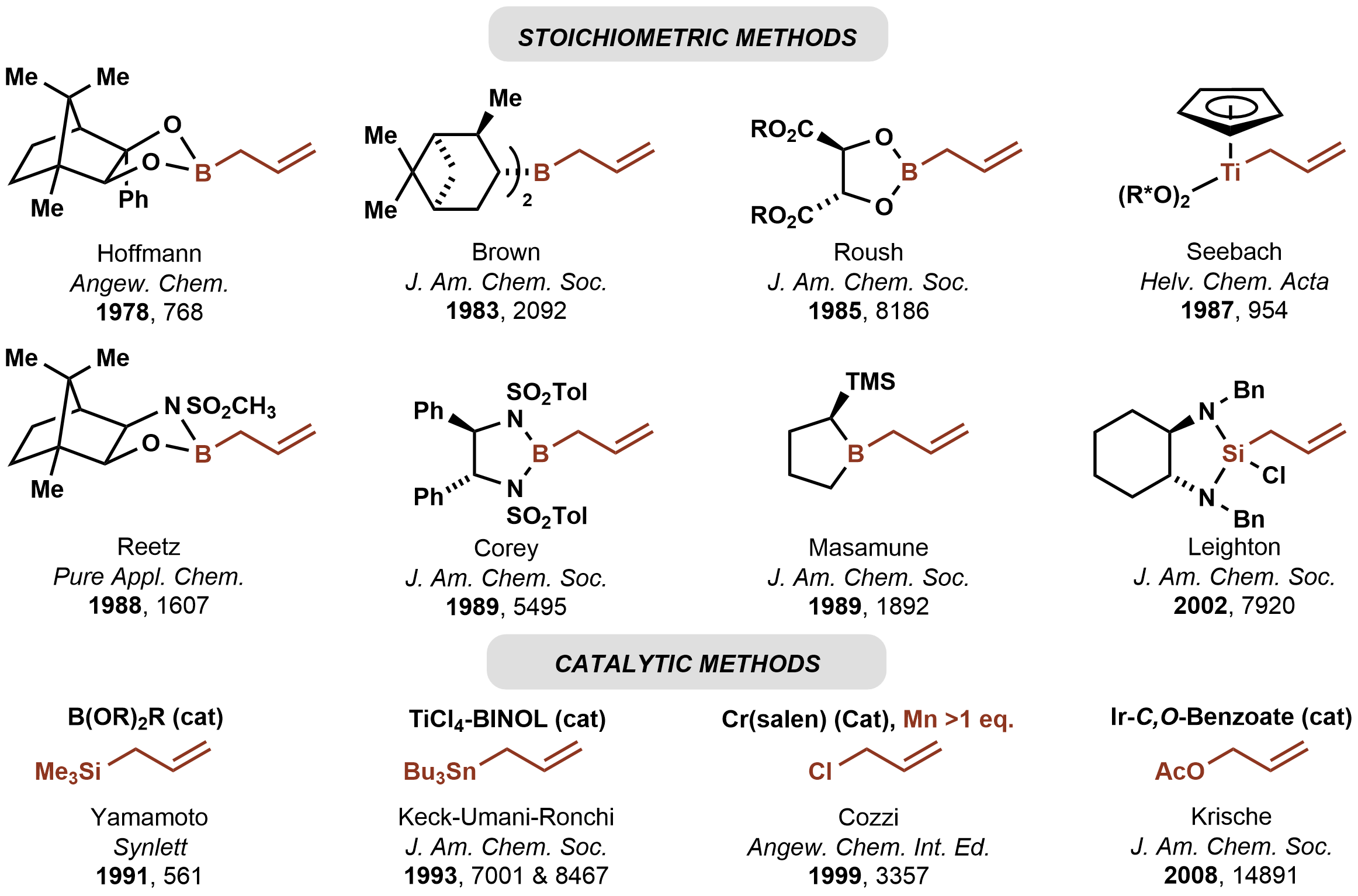
In 1978, Hoffmann reported the first asymmetric carbonyl allylation using achiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
allylmetal reagent, an allylborane derived from camphor
Camphor () is a waxy, colorless solid with a strong aroma. It is classified as a terpenoid and a cyclic ketone. It is found in the wood of the camphor laurel (''Cinnamomum camphora''), a large evergreen tree found in East Asia; and in the kapu ...
. Such methods utilize preformed allyl metal reagents. The approach is well developed using allyl boranesDenmark, S. E.; Almstead, N. G. In ''Modern Carbonyl Chemistry''; Otera, J., Ed.; Wiley-VCH: Weinheim, 2000; Chapter 10.
''(13)'' As illustrated by the Keck allylation, catalytic enantioselective additions of achiral allylmetal reagents to carbonyl compounds also are possible by
As illustrated by the Keck allylation, catalytic enantioselective additions of achiral allylmetal reagents to carbonyl compounds also are possible by organostannane addition Organostannane addition is reaction involving the nucleophilic addition of an allyl-, allenyl-, or propargyl- stannane to an aldehyde, imine, or (in rare cases) a ketone. This reaction is widely used for carbonyl allylation.
The addition of an ...
s.
Allylic boronate and -borane reagents have also been developed for enantioselective addition to carbonyls—in this class of reactions, the allylic boron reagent confers stereochemical control
''(13)''
Catalysis
In 1991, Yamamoto disclosed the first catalytic enantioselective method for carbonyl allylation, which employed a chiral boron Lewis acid-catalyst in combination with allyltrimethylsilane. Numerous other catalytic enantioselective methods for carbonyl allylation followed. Catalytic variants of the Nozaki-Hiyama-Kishi reaction represent an alternative method for asymmetric carbonyl allylation, but stoichiometric metallic reductants are required. Whereas the aforementioned asymmetric carbonyl allylations rely on preformed allylmetal reagents, theKrische allylation
The Krische allylation involves the enantioselective iridium-catalyzed addition of an allyl group to an aldehyde or an alcohol (chemistry), alcohol, resulting in the formation of a secondary homoallylic alcohol. The mechanism of the Krische allylat ...
exploits allyl acetate
Allyl acetate is an organic compound with formula C3H5OC(O)CH3. This colourless liquid is a precursor to especially allyl alcohol, which is a useful industrial intermediate. It is the acetate ester of allyl alcohol.
Preparation
Allyl acetate is ...
for enantioselective carbonyl allylation. Selected methods for asymmetric carbonyl allylation are summarized below.

Use in total synthesis
Carbonyl allylation has been employed in the synthesis ofpolyketide
In organic chemistry, polyketides are a class of natural products derived from a Precursor (chemistry), precursor molecule consisting of a Polymer backbone, chain of alternating ketone (, or Carbonyl reduction, its reduced forms) and Methylene gro ...
natural products and other oxygenated molecules with a contiguous array of stereocenters. For example, allylstannanation of a threose-derived aldehyde affords the macrolide
Macrolides are a class of mostly natural products with a large macrocyclic lactone ring to which one or more deoxy sugars, usually cladinose and desosamine, may be attached. Macrolides belong to the polyketide class of natural products. ...
antascomicin B, which structurally resembles FK506 and rapamycin
Sirolimus, also known as rapamycin and sold under the brand name Rapamune among others, is a macrolide compound that is used to coat coronary stents, prevent organ rejection, organ transplant rejection, treat a rare lung disease called lymphang ...
, and is a potent binder of FKBP12. The Krische allylation
The Krische allylation involves the enantioselective iridium-catalyzed addition of an allyl group to an aldehyde or an alcohol (chemistry), alcohol, resulting in the formation of a secondary homoallylic alcohol. The mechanism of the Krische allylat ...
was used to prepare the polyketide (+)-SCH 351448, a macrodiolide ionophore bearing 14 stereogenic centers.

Older primary literature
* * * * * * *References