Bottom-blown oxygen converter on:
[Wikipedia]
[Google]
[Amazon]
 The Bottom-blown Oxygen Converter or BBOC is a smelting furnace developed by the staff at Britannia Refined Metals Limited (“BRM”), a British subsidiary of MIM Holdings Limited (which is now part of the Glencore group of companies). The furnace is currently marketed by Glencore Technology. It is a sealed, flat-bottomed furnace mounted on a tilting frame that is used in the recovery of precious metals. A key feature is the use of a shrouded lance to inject oxygen through the bottom of the furnace, directly into the precious metals contained in the furnace, to oxidize base metals or other impurities as part of their removal as slag.J M Floyd, “Submerged bath smelting applied to the non-ferrous metal industry,” in: ''The Paul E. Queneau International Symposium, Extractive Metallurgy of Copper, Nickel and Cobalt, Volume I: Fundamental Aspects'', Eds R G Reddy and R N Weizenbach (The Minerals, Metals and Materials Society: Warrendale, Pennsylvania, 1993), 473–488.
The Bottom-blown Oxygen Converter or BBOC is a smelting furnace developed by the staff at Britannia Refined Metals Limited (“BRM”), a British subsidiary of MIM Holdings Limited (which is now part of the Glencore group of companies). The furnace is currently marketed by Glencore Technology. It is a sealed, flat-bottomed furnace mounted on a tilting frame that is used in the recovery of precious metals. A key feature is the use of a shrouded lance to inject oxygen through the bottom of the furnace, directly into the precious metals contained in the furnace, to oxidize base metals or other impurities as part of their removal as slag.J M Floyd, “Submerged bath smelting applied to the non-ferrous metal industry,” in: ''The Paul E. Queneau International Symposium, Extractive Metallurgy of Copper, Nickel and Cobalt, Volume I: Fundamental Aspects'', Eds R G Reddy and R N Weizenbach (The Minerals, Metals and Materials Society: Warrendale, Pennsylvania, 1993), 473–488.
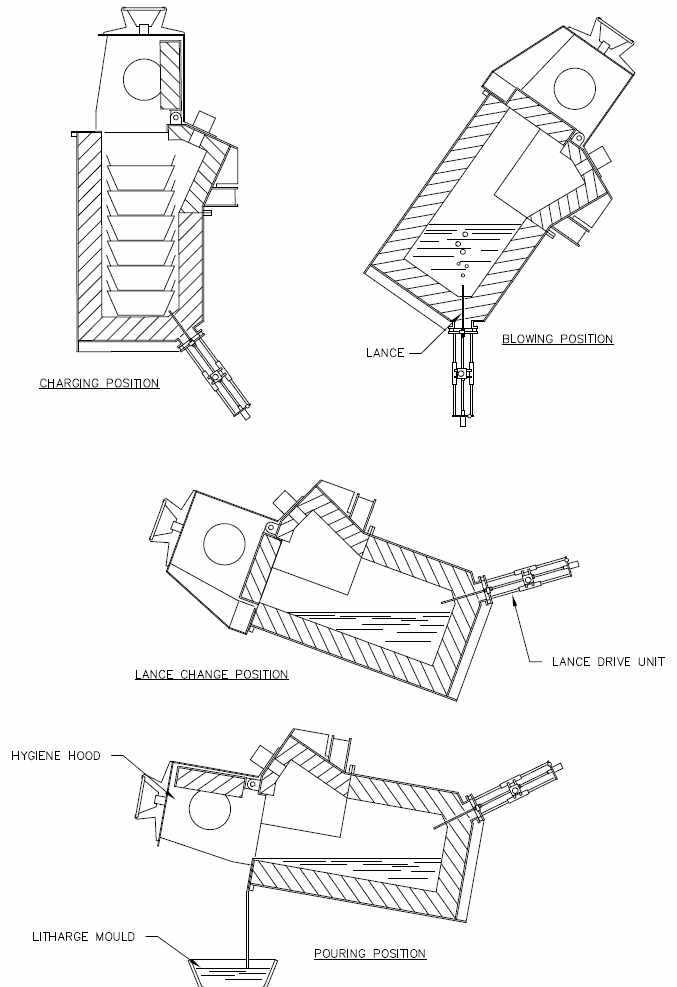 Figure 2 shows the positions of the BBOC at various stages of the operating cycle.
The BBOC is held in an upright position during the charging stage. A solid or liquid charge is added using an overhead crane. The furnace is then tilted forward so that the lance is above the charge, and the charge is melted using an oil or natural gas burner that is inserted near the top of the furnace. Once the charge has been melted, the furnace is tilted back into the blowing position and oxygen is blown into the bath. Slag formed from the oxidation of lead and zinc is removed periodically by tilting the furnace forward again and pouring it off.
The oxygen flow rate during blowing for a three tonne capacity furnace is 20–30 Nm3/h. Zinc is initially oxidized to form a
Figure 2 shows the positions of the BBOC at various stages of the operating cycle.
The BBOC is held in an upright position during the charging stage. A solid or liquid charge is added using an overhead crane. The furnace is then tilted forward so that the lance is above the charge, and the charge is melted using an oil or natural gas burner that is inserted near the top of the furnace. Once the charge has been melted, the furnace is tilted back into the blowing position and oxygen is blown into the bath. Slag formed from the oxidation of lead and zinc is removed periodically by tilting the furnace forward again and pouring it off.
The oxygen flow rate during blowing for a three tonne capacity furnace is 20–30 Nm3/h. Zinc is initially oxidized to form a
Accessed 23 August 2013.
 The Bottom-blown Oxygen Converter or BBOC is a smelting furnace developed by the staff at Britannia Refined Metals Limited (“BRM”), a British subsidiary of MIM Holdings Limited (which is now part of the Glencore group of companies). The furnace is currently marketed by Glencore Technology. It is a sealed, flat-bottomed furnace mounted on a tilting frame that is used in the recovery of precious metals. A key feature is the use of a shrouded lance to inject oxygen through the bottom of the furnace, directly into the precious metals contained in the furnace, to oxidize base metals or other impurities as part of their removal as slag.J M Floyd, “Submerged bath smelting applied to the non-ferrous metal industry,” in: ''The Paul E. Queneau International Symposium, Extractive Metallurgy of Copper, Nickel and Cobalt, Volume I: Fundamental Aspects'', Eds R G Reddy and R N Weizenbach (The Minerals, Metals and Materials Society: Warrendale, Pennsylvania, 1993), 473–488.
The Bottom-blown Oxygen Converter or BBOC is a smelting furnace developed by the staff at Britannia Refined Metals Limited (“BRM”), a British subsidiary of MIM Holdings Limited (which is now part of the Glencore group of companies). The furnace is currently marketed by Glencore Technology. It is a sealed, flat-bottomed furnace mounted on a tilting frame that is used in the recovery of precious metals. A key feature is the use of a shrouded lance to inject oxygen through the bottom of the furnace, directly into the precious metals contained in the furnace, to oxidize base metals or other impurities as part of their removal as slag.J M Floyd, “Submerged bath smelting applied to the non-ferrous metal industry,” in: ''The Paul E. Queneau International Symposium, Extractive Metallurgy of Copper, Nickel and Cobalt, Volume I: Fundamental Aspects'', Eds R G Reddy and R N Weizenbach (The Minerals, Metals and Materials Society: Warrendale, Pennsylvania, 1993), 473–488.
Introduction
Ores mined for their base metal content often containprecious metal
Precious metals are rare, naturally occurring metallic chemical elements of high Value (economics), economic value. Precious metals, particularly the noble metals, are more corrosion resistant and less reactivity (chemistry), chemically reac ...
s, usually gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
and silver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. ...
. These have to be removed from the base metals as part of the refining processes used to purify the metals. In the case of copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
electrolytic refining, the gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
and silver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. ...
fall to the bottom of the electrolytic refining cell as “ slimes” that are subsequently treated to recover gold and silver as byproducts. In the case of lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
refining, silver, gold, and other precious metals are typically removed using the Parkes process
The Parkes process is a pyrometallurgical industrial process for removing silver from lead during the production of bullion. It is an example of liquid–liquid extraction.
The process takes advantage of two liquid-state properties of zinc. The fi ...
, in which zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
is added to the impure lead bullion to collect the silver, gold and other precious metals.
The BRM lead refinery at Northfleet
Northfleet is a town in the borough of Gravesham in Kent, England. It is located immediately west of Gravesend, and on the border with the Borough of Dartford. Northfleet has its own railway station on the North Kent Line, just east of Ebbsf ...
in England uses the Parkes process followed by liquation and a vacuum induction retort to recover precious metals. The product of this process is a feed for the BBOC consisting of a mixture of lead, silver (60–75%), zinc (2–3%) and copper (2–3%), with trace amounts of gold.R P Knight, “Oxidation refining in the Bottom Blown Oxygen Converter,” ''Erzmetall'', 48 (8), 1995, 530–537. Prior to the development of the BBOC, BRM used cupellation
Cupellation is a refining process in metallurgy in which ores or alloyed metals are treated under very high temperatures and subjected to controlled operations to separate noble metals, like gold and silver, from base metals, like lead, co ...
in a 15 tonne (“t”) reverberatory cupellation furnace to recover the precious metals from this mixture. Three of these furnaces were used to produce 450 t of silver per year.
Cupellation works by exposing the mixture at high temperature to air or oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
. The base metals, being less noble
A noble is a member of the nobility.
Noble may also refer to:
Places Antarctica
* Noble Glacier, King George Island
* Noble Nunatak, Marie Byrd Land
* Noble Peak, Wiencke Island
* Noble Rocks, Graham Land
Australia
* Noble Island, Gr ...
than silver and gold, react with the oxygen to form their oxides, which separate from the noble metals to form a slag that floats on the top of the residual metals (or “ doré”). At BRM, the doré contains 99.7% silver.
To maximize the oxygen transfer from the blast air in the reverberatory furnace, a shallow bath is used, thus increasing the surface-area-to-volume ratio of the furnace.R P Knight, “Further applications of the bottom blown oxygen converter,” in: ''International Symposium on Injection in Process Metallurgy'', Eds T Lehner, P J Koros and V Ramachandran (The Minerals, Metals and Materials Society: Warrendale, Pennsylvania, 1991), 335–346.
A problem with using reverberatory furnaces for cupellation is that the zinc oxidizes first, forming a crust across the top of the molten material. This crust prevents the penetration of oxygen to the rest of the material, and so it has to be manually broken up and removed using a rabble
Rabble may refer to:
* Hoi polloi, a negative term for the common people
* rabble.ca, a Canadian website
* An arrow in the arcade game '' Libble Rabble''
* Rabble of Devilkin, characters in the ''Dungeons & Dragons'' roleplaying game
* '' Rabble ...
bar. This is both labor-intensive and also results in the loss of some of the silver. Similarly, the oxidized lead slag has to be removed when it forms to maintain the operation, and its removal also results in loss of silver.
The BBOC was developed by BRM personnel as a way of reducing these and other problems, such as low energy efficiency and low oxygen utilization, associated with the reverberatory cupellation process.
Description
The BBOC furnace is a cylindrical steel vessel with a protective internal lining of refractory bricks. It is mounted on a tilting frame that allows it to be held at different angles at different stages of its operating cycle (see Figure 2). A hood is fixed over the top of the furnace, providing a seal that prevents lead and other fumes from escaping during the furnace’s operation (see Figure 1). The key feature of the BBOC is the shrouded lance that passes through the refractory bricks at the bottom of the furnace. This lance allows oxygen to be injected directly into the molten metal contained in the furnace, away from the refractory lining. Doing so allows the region of high reaction rates to be removed from the vicinity of the lining, thus reducing its wear. By injecting the oxygen directly into the bath, rather than blowing it on top (as in the case of the reverberatory cupellation furnace or top-blown rotary converters), the oxygen transfer efficiency is not impeded by the presence of the slag layer. It results in an oxygen utilization efficiency approaching 100%. The lack of interference in the oxygen transfer by the slag layer has a couple of key benefits. The first is that the increased certainty in the estimation of oxygen utilization efficiency means that it is easier to calculate the endpoint of the process, making process control much easier. The second is that a thicker slag layer can be tolerated (because the oxygen does not have to pass through it), and this means that the losses of silver to the slag are reduced (because it is the silver at the interface between the metal and slag that becomes entrained during the removal of the slag and the thicker the slag layer, the smaller the silver content of the removed slag). BRM reported a decrease in the silver content of the BBOC slag compared to the reverberatory furnace slag of 50%. BRM found that the reaction rate of the BBOC was 10–20 times that of its reverberatory cupellation furnace. Refractory wear in the BBOC is largely confined to the slag line, at the top of the metal, where attack bylitharge
Litharge (from Greek , 'stone' + 'silver' ) is one of the natural mineral forms of lead(II) oxide, PbO. Litharge is a secondary mineral which forms from the oxidation of galena ores. It forms as coatings and encrustations with internal tetr ...
(lead oxide
Lead oxides are a group of inorganic compounds with formulas including lead (Pb) and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), gr ...
) is greatest. This is combated by using fused-grain, direct-bonded magnesite-chrome bricks to line the inside of the furnace shell.
Operation
 Figure 2 shows the positions of the BBOC at various stages of the operating cycle.
The BBOC is held in an upright position during the charging stage. A solid or liquid charge is added using an overhead crane. The furnace is then tilted forward so that the lance is above the charge, and the charge is melted using an oil or natural gas burner that is inserted near the top of the furnace. Once the charge has been melted, the furnace is tilted back into the blowing position and oxygen is blown into the bath. Slag formed from the oxidation of lead and zinc is removed periodically by tilting the furnace forward again and pouring it off.
The oxygen flow rate during blowing for a three tonne capacity furnace is 20–30 Nm3/h. Zinc is initially oxidized to form a
Figure 2 shows the positions of the BBOC at various stages of the operating cycle.
The BBOC is held in an upright position during the charging stage. A solid or liquid charge is added using an overhead crane. The furnace is then tilted forward so that the lance is above the charge, and the charge is melted using an oil or natural gas burner that is inserted near the top of the furnace. Once the charge has been melted, the furnace is tilted back into the blowing position and oxygen is blown into the bath. Slag formed from the oxidation of lead and zinc is removed periodically by tilting the furnace forward again and pouring it off.
The oxygen flow rate during blowing for a three tonne capacity furnace is 20–30 Nm3/h. Zinc is initially oxidized to form a zinc oxide
Zinc oxide is an inorganic compound with the Chemical formula, formula . It is a white powder which is insoluble in water. ZnO is used as an additive in numerous materials and products including cosmetics, Zinc metabolism, food supplements, rubbe ...
dross
Dross is a mass of solid impurities floating on a molten metal or dispersed in the metal, such as in wrought iron. It forms on the surface of low- melting-point metals such as tin, lead, zinc or aluminium or alloys by oxidation of the metal. Fo ...
on the surface of the charge, but as lead oxide subsequently forms, a fluid slag of zinc and lead oxides is created. Most of the copper is removed at the same time as the lead. The final removal of copper to a level of 0.04% is undertaken at the end of the process by further additions of lead to collect the copper.
If the lance needs to be replaced at any time during operation, this is done by tilting the furnace forward until the lance is above the surface of the bath, where it can be removed and replaced without the contents of the furnace draining through the hole in the furnace lining.
The cupellation process finishes when the silver is about 99.7% pure. At this point, the silver is poured from the furnace and transferred to another furnace, where a flux is added to upgrade and remove the oxygen from the silver to produce market bullion of 99.9% purity.
History
Early development at BRM
Staff at BRM began work on an alternative to the conventional reverberatory cupellation furnace in the early 1980s. This included a review of the available technology, including the top-blown rotary converter ("TBRC"), on which test work was undertaken. One of the first areas investigated was the use of oxygen-enriched blast air in the reverberatory furnace. This was “found to be of marginal benefit and not economically viable." The BRM staff subsequently tried to increase the oxygen transfer rate by using lances submerged in the bath of the reverberatory furnace and found that there was some benefit in doing this. However, the wear rate of the lances was excessive and it was realized that the basic design of the furnace, with its shallow bath, was not conducive to the development of a high-intensity reactor. The concept then evolved into a new furnace design, one that had a deep bath, in contrast to the reverberatory furnace design. Initial tests of the bottom injection of oxygen were carried out on a small scale at Imperial College, London, using a nitrogen-shrouded tuyere.K R Barrett, “Operation of the bottom blown oxygen cupel at Britannia Refined Metals, Ltd,” in: ''Today's Technology for the Mining and Metallurgical Industry, MMIJ/IMM Joint Symposium 1989, Kyoto, Japan, 2–4 October 1989,'' (The Mining and Materials Processing Institute of Japan, and the Institution of Mining and Metallurgy: 1989), 589–595. These showed that under certain conditions a protective accretion would form at the tip of the injector, and that oxygen utilization was high, with the oxidation reactions generating sufficient heat to keep the furnace hot until the final stages of refining when the impurity levels were low. Additionally, the test work on the TBRC had shown that it had a high rate of refractory wear, due to the washing action of the slag caused by the rotation of the furnace, which provided additional pressure to develop an alternate process. The TBRC test work also resulted in low oxygen utilization (about 60%). Based on the success of the small-scale tests, and with calculations indicating that the new design would have significant energy savings over the reverberatory furnace, the BRM staff built a 1.5 t pilot plant with a working volume of 150 liters (“L”). The oxygen injector was a fixed tuyere, located at corner of the base with the side wall, with an annular nitrogen shroud. The initial pilot plant tests showed that it was difficult to maintain the protective accretion that had been generated in the small-scale tests, due to the variation in temperature and bullion composition that occurred throughout the cupelling cycle. Without the accretion, the nitrogen shroud could not provide sufficient protection to the injector, and it burned back to the level of the refractory lining, which resulted in damage to the lining. The solution eventually developed was the concept of the moveable lance system in place of the fixed tuyere that had been used initially. The lance was pushed further into the furnace as its tip was worn away. The initial lance advancing system was manual, but the current automated system was subsequently developed. Once a sustainable system had been developed in the pilot plant, and after three years of pilot plant development, a commercial, 3 t-scale BBOC was commissioned at BRM in 1986. Its use reduced the fuel consumption per tonne of silver by 85%, from 30 gigajoules per tonne (“GJ/t”) to 4.5 GJ/t and the exhaust gas volume from 32 000 Nm3/h to 7500 Nm3/h.Commercialization
After the successful operation of the BBOC at BRM, MIM Holdings Limited (“MIM”) decided to license the technology to other smelter and refinery operators. Early adopters included Hindustan Zinc Limited, which by 1995 had two 1 t BBOC plants operating in India, and ASARCO Inc., which was operating a 3 t BBOC furnace at its Omaha, Nebraska, refinery.Rand Refinery
The South African companyRand Refinery
Rand Refinery (Pty) Limited is the world's largest integrated single-site precious metals refining and smelting complex. It was established in 1920 to refine gold within South Africa, which had previously been refined in London.
History
It was ...
Limited rebuilt its smelter in 1986, incorporating two 1.5 t TBRCs and a small static reverberatory furnace for cupellation to produce doré bullion containing gold and silver.M Griffin, “Change from top-blown to bottom-blown converter for lead bullion cupellation at Rand Refinery,” in: ''Pyrometallurgy ’95'' (Institution of Mining and Metallurgy: London, 1995), 65–87. . The original concept was to produce doré bullion directly from the TBRCs, but this proved impossible, as it was found impossible to take the oxidation stage to completion while maintaining temperatures at which the doré would remain molten. Consequently, the reverberatory cupellation furnace was necessary to complete the process.
In January 1993, the management team of Rand Refinery decided to review alternate technologies to replace the TBRC–reverberatory furnace circuit, with the objective of having cupellation undertaken in a single stage. After evaluating the possibility of modifying the existing TBRCs by replacing the existing lance–burner combination with a separate lance and burner, and considering complete replacement of the TBRCs with an Ausmelt top-submerged lance furnace, Rand Refinery decided to replace one of the TBRC with a 4 t BBOC. The remaining TBRC is used to treat litharge slag to recover the lead for sale.
The Rand Refinery BBOC was commissioned in 1994. The operators reported a 28% reduction in the operating costs when the BBOC’s costs were compared with those of the TBRC–reverberatory furnace combination. This included a 45% reduction in bulk oxygen costs and halving the number of operators required to run the plant. The BBOC’s refractory life was 13 weeks, compared to an average refractory life of 2 weeks for the TBRCs. Other maintenance costs also fell.
Broken Hill Associated Smelters
The Broken Hill Associated Smelters Proprietary Limited (“BHAS”) lead smelter, now owned byNyrstar
Nyrstar is an international producer of minerals and metals. It was founded in August 2007 and listed on the Euronext Brussels that October.
Nyrstar has mining, smelting and other operations located in Europe, the United States and Australia an ...
NV, has been the world’s largest lead smelter. Its staff was responsible for many significant technical developments in the lead smelting industry, including the updraft sinter plant and continuous lead refining.
Until 1990, BHAS recovered silver in a two-stage reverberatory cupellation process.A Mills, G C Burgess and D Thompson, “Development of intensive doré silver cupellation at Pasminco Metals – BHAS,” in: ''Extractive Metallurgy of Gold and Base Metals'', Kalgoorlie, 26–28 October 1992 (The Australasian Institute of Mining and Metallurgy: Melbourne, 1992), 465–469. This process suffered from low recoveries (80–83%), a long cycle time (4–5 days) that caused large in-process inventories, inefficient use of labor and energy, and poor workplace hygiene.D Swinbourne, A Winters and M Giunti, “Theory and practice of cupellation at Port Pirie Pasminco smelter,” in: ''European Metallurgical Conference EMC 2001, Friedrichshafen, Germany, 18–21 September 2001, Volume 3: Light Metals, Process Control, Analytics and Modelling, Education and Training, Precious and Rare Metals'', Eds F Liese and U Waschki (GDMB-Informationsgesellschaft GmbH: Clausthal-Zellerfeld, 2001), 329–345. . After a test work program undertaken at Ausmelt’s facilities in Melbourne, BHAS switched to using a process based on the Sirosmelt top-submerged lance in June 1990.
The change to the lance-based furnace increased oxygen utilization to 95% and the cycle time was reduced to a little less than eight hours, “but the grade of the doré which could be economically produced was poor.” The doré from the new furnace still contained about 0.8% lead and 0.4% copper. It was also found impractical to cast anode plates of doré directly from the Sirosmelt furnace, so the Sirosmelt doré had to undergo a further refining step in a reverberatory furnace, together with a sodium nitrate flux.
Then, in 1996, BHAS decided to modernize the refining circuit and replaced the Sirosmelt silver refining furnace with a BBOC furnace.P Kapoulitsas, M Giunti, R Hampson, A Cranley, S Gray, B Kretschmer, R Knight and J Clark “Commissioning and optimisation of the new lead and silver refinery at the Pasminco Port Pirie Smelter,” in: ''Lead–Zinc 2000'', Eds J E Dutrizac, J A Gonzalez, D M Henke, S E James and A H-J Siegmund (The Minerals, Metals and Materials Society: Warrendale, Pennsylvania, 2000), 187–201. . Commissioning of the modernized refining circuit was completed in 1999, and the lead throughput was increased by 11%, with the silver refining capacity increasing to over 400 t/y.
The BBOC process proved to be “generally successful”, although it did suffer some problems with the lance jamming that were attributed to higher than expected levels of zinc in the feed, due to problems removing the zinc in earlier stages of the refinery circuit. The higher levels of zinc also caused higher than expected refractory wear and excessive lance consumption, because the heat generated by oxidizing the zinc was greater than that of oxidizing lead.
The BBOC furnace proved to be capable of producing doré containing as little as 0.01% lead and less than 0.1% copper at a temperature around 1050 °C, but BHAS wanted to cast the doré directly into anode plates using an existing doré casting conveyor. Casting using the existing conveyor proved impossible at an operating temperature
An operating temperature is the allowable temperature range of the local ambient environment at which an electrical or mechanical device operates. The device will operate effectively within a specified temperature range which varies based on the de ...
of 1050 °C, because the high thermal conductivity of the silver resulted in it freezing before it reached the molds. Consequently, BHAS decided to increase the operating temperature to 1100–1150 °C so that the silver remained liquid until cast into the anode molds. A side effect of this is that the lead and copper content of the product doré are higher than if the furnace is operated at 1050 °C, at 0.2% lead and 0.6% copper. Thermodynamic calculations have shown that this is unavoidable at this higher operating temperature.
Other lead smelters
Besides the smelters named so far, the BBOC has been licensed to the operators of the Trail smelter in British Columbia, the Belledune smelter in New Brunswick, the Noyelles Godault smelter in France, the Korea Zinc zinc smelter in Onsan, South Korea, and the lead smelter at Chanderiya in India.BBOC™ – Minimising metal inventory.Accessed 23 August 2013.
Other applications
In addition to its use in recovering silver in lead refineries, the BBOC has been used to treat anode slimes from copper electrolytic refineries. Anode slimes are composed of solid particles that do not dissolve in theelectrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
in the refining cells. This includes the gold and silver present in the copper anodes that are being refined. As with recovering silver in lead smelting, reverberatory furnaces are often used in the copper refining industry for the purification and recovery of gold and silver from anode slimes.P D Parker, J A Bonucci and J E Hoffmann, “Recovery of high purity silver from sulfated copper refinery slimes,” in: ''Hydrometallurgical Processes for Byproduct Recovery'' (Society of Mining Engineers: Littleton, Colorado, 1981), 177–184. .W Charles Cooper, “The treatment of copper refinery anode slimes,” ''JOM'', August 1990, 45–49. However, the reverberatory furnaces suffer from similar disadvantages in copper anode doré production as they do in lead refineries,G G Barbante, D R Swinbourne and W J Rankin, “Pyrometallurgical treatment of tank house slimes,” in: ''Pyrometallurgy for Complex Minerals & Wastes'', Eds M Nilmani, T Lehner and W J Rankin (The Minerals, Metals and Materials Society: Warrendale, Pennsylvania, 1994), 319–337. including resulting in a large inventory of gold in the system. Other furnace types used, include top-blown rotary converters and short rotary furnaces.
ASARCO Amarillo copper refinery
The ASARCO Amarillo copper refinery switched in 1991 from reverberatory furnace treatment of anode slimes to a BBOC to reduce the gold inventory. The original reverberatory furnace had a 15 t capacity. The production cycle of the reverberatory furnace was typically 7–10 days, with the final doré production being about 8 t per cycle. A single 3 t capacity BBOC was installed, and it was found to increase rejection of selenium from the slimes, with a reduction in fluxing requirements of about 80%.Sumitomo Metal Mining Niihama refinery
In the 1990s, the Niihama copper refinery, owned by Sumitomo Metal Mining Company Limited (“Sumitomo”), treated copper anode slimes generated in-house, together with anode slimes from Sumitomo’s Toyo refinery and lead refinery slime from the Harima Imperial Smelting Process smelter.C Segawa and T Kusakabe, “Current operations in SMM’s slime treatment,” in: ''EPD Congress 1996'', Ed. G W Warren (The Minerals, Metals and Materials Society: Warrendale, Pennsylvania, 1995), 43–52. A total of 1200 tonnes per year (“t/y”) of anode slimes and 400 t/y of lead refinery slimes were treated using a process flow sheet that included a chloridizing step to remove separate the lead as lead chloride (PbCl2) and a reverberatory-type doré furnace. It produced about 200 t of silver, 22 t of gold, 1.5 t ofpalladium
Palladium is a chemical element; it has symbol Pd and atomic number 46. It is a rare and lustrous silvery-white metal discovered in 1802 by the English chemist William Hyde Wollaston. He named it after the asteroid Pallas (formally 2 Pallas), ...
, 300 kilograms (“kg”) of platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
and 40 kg of rhodium
Rhodium is a chemical element; it has symbol Rh and atomic number 45. It is a very rare, silvery-white, hard, corrosion-resistant transition metal. It is a noble metal and a member of the platinum group. It has only one naturally occurring isot ...
, as well as 60 t of selenium
Selenium is a chemical element; it has symbol (chemistry), symbol Se and atomic number 34. It has various physical appearances, including a brick-red powder, a vitreous black solid, and a grey metallic-looking form. It seldom occurs in this elem ...
, 50 t of bismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...
, 900 kg of tellurium
Tellurium is a chemical element; it has symbol Te and atomic number 52. It is a brittle, mildly toxic, rare, silver-white metalloid. Tellurium is chemically related to selenium and sulfur, all three of which are chalcogens. It is occasionally fou ...
and 150 t of antimony
Antimony is a chemical element; it has chemical symbol, symbol Sb () and atomic number 51. A lustrous grey metal or metalloid, it is found in nature mainly as the sulfide mineral stibnite (). Antimony compounds have been known since ancient t ...
alloy annually.
The gold production doubled during the decade to 1996, as its concentration in anode slimes and the quantity of anode slimes increased. To enable this, Sumitomo decided in 1990 to upgrade the refinery, and as part of that upgrade, installed a 3.5 t-capacity BBOC to replace its reverberatory doré furnace in October 1992.
Sumitomo reported that, while the old oil-fired reverberatory furnace had served it well for many years, it had the following drawbacks:
* its operation was labor-intensive
* it had a low fuel efficiency
* there was a high waste gas volume
* the reaction rate was low.
Sumitomo investigated both the TBRC and BBOC furnaces before making a selection. It chose the BBOC over the TBRC technology because of the ease of control of the bath temperature, its high oxygen efficiency and its simple maintenance.
Sumitomo found that the impurity contents of BBOC doré anodes was high when the furnace was first commissioned. This was because it was important to determine the endpoint of the oxidation reactions to maximize the quality of the anodes. Sumitomo found that this could be determined by measuring the oxygen content of the off-gas using oxygen sensors based on stabilized zirconia
Zirconium dioxide (), sometimes known as zirconia (not to be confused with zirconium silicate or zircon), is a white crystalline oxide of zirconium. Its most naturally occurring form, with a monoclinic crystalline structure, is the mineral ba ...
with an Fe/FeO reference electrode.
Sumitomo subsequently adapted the BBOC to allow the chloridizing step to be undertaken in the furnace, thus eliminating the need for a separate chloridizing furnace for lead chloride production. This was done in February 1994 and was reported to be “giving very good results.”
Takehara copper refinery
The Takehara copper refinery of the Mitsui Mining & Smelting Company Limited of Japan commissioned a BBOC in its precious metals department in 1993. Prior to the installation of the BBOC, the Takehara refinery refined a mixture of copper and lead anode slimes in a three reverberatory furnaces (two operating and one being rebricked) in a process that had a cycle time of 104 hours for refining 6 t of bullion. The reverberatory furnaces were replaced with a single BBOC with a charge capacity of 6 t of feed. The cycle time was reduced to 50 hours. The use of the BBOC reduced the energy consumption from 74 GJ/t to 27 GJ/t and also had betterbismuth
Bismuth is a chemical element; it has symbol Bi and atomic number 83. It is a post-transition metal and one of the pnictogens, with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth occurs nat ...
elimination than the reverberatory furnaces.
Advantages
The following advantages have been reported for the BBOC: * ''very high oxygen efficiency'' – the injection of oxygen directly into the reaction zone within the furnace results in much greater oxygen efficiency (close to 100%) than with reverberatory furnaces (8% for the Niihama furnace) or top-blown rotary converters (about 30%) * ''reduced off-gas volume'' – the use of industrial oxygen and the high oxygen efficiency of the process means that excess air is not required to achieve the results. This reduces the off-gas volume and thus the cost of the off-gas train and handling equipment. Rand Refinery reported that the off-gas volume of the BBOC was about 75% of that of a TBRC with a special lance conversion and only 19% of that of top-submerged lance smelting. Niihama refinery reported that its BBOC had 15% of the off-gas volume of its reverberatory furnace while producing 1.8 times the product * ''higher reaction rates'' – by injecting the oxygen directly into the reaction zone, the reaction rates are much higher than in reverberatory furnaces where the oxygen has first to penetrate the slag layer. BRM reported a reaction rate per unit of furnace volume of 10–20 times that of the reverberatory furnace * ''lower refractory wear'' – Rand Refinery reported that the refractory linings of its TBRC furnaces needed replacing after approximately two weeks, while the linings of its BBOC furnace lasted about 14 weeks * ''lower precious metal inventories'' – a consequence of the higher reaction rates is that smaller furnace volumes are required and there are smaller cycle times. This results in lower precious metal inventories. In lead slimes bullion processing, the silver inventory was reduced from 4.5 t to 1.25 t after replacing a reverberatory furnace with a BBOC and at BRM the silver inventory fell from 11.5 t to 3.1 t with the introduction of the BBOC furnace * ''better energy efficiency'' – a supplementary burner is needed only during heating the charge and doré casting operations. During cupellation, the oxidation reactions provide sufficient heat to maintain temperature. There was a 92% reduction in fuel consumption per tonne of doré treated reported for the BBOC at the Niihama refinery * ''better product quality'' – BHAS reported that lead and copper levels in silver produced from the BBOC of 0.01% and 0.1% respectively were possible when the furnace was operating under design conditions, compared to 0.04% and 0.2% for the old reverberatory furnace, and 0.8% and 0.4% for the Sirosmelt furnace. Rand Refinery reported that a doré bullion of 99.2% was achievable. BRM reported that its doré is 99.7% silver * ''higher recoveries of precious metals'' – due to changes in the way the BBOC is operated compare to reverberatory furnaces, notably in being able to use deeper layers of slag, there is an increase in the recovery of precious metals compared to the reverberatory furnaces. Replacement of reverberatory furnaces with BBOC furnaces saw the direct silver recovery increase from 92.5% to 97.5% at BRM and from 70% to over 95% at Niihama * ''simple vessel design'' – the BBOC has a relatively simple vessel design, without the complex moving parts of TBRCs * ''good process control'' – the high oxygen utilization allows good process control, particularly when combined with an oxygen sensor in the off-gas system * ''lower labor requirements'' – the BBOC has a lower labor requirement than reverberatory furnaces, top-submerged lance furnaces and TBRCs * ''lower operating costs'' – lower labor requirements, lower fuel requirements and longer refractory life contributed to a 28.3% reduction in overall operating costs when the BBOC was installed at the Rand Refinery * ''lower capital cost'' – the BBOC is a simpler furnace than TBRC or top-submerged lance furnaces. Rand Refinery reported a capital cost comparison indicating that its BBOC option was 67% of the cost of a top-submerged lance option.References
{{Extractive metallurgy Metallurgy Smelting Metallurgical processes Industrial furnaces