Aluminylene on:
[Wikipedia]
[Google]
[Amazon]
 Aluminylenes are a sub-class of
Aluminylenes are a sub-class of
 Power's aluminylene was shown to react with organic
Power's aluminylene was shown to react with organic  The N-aluminylene reported by Liu and coworkers was shown to undergo an oxidative insertion reaction when mixed with IDippCuCl (IDipp=1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) to form a terminal
The N-aluminylene reported by Liu and coworkers was shown to undergo an oxidative insertion reaction when mixed with IDippCuCl (IDipp=1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) to form a terminal  Liu also demonstrated that the N-aluminylene could act as an important precursor to
Liu also demonstrated that the N-aluminylene could act as an important precursor to  In 2023, Liu and coworkers published further examples of the reactivity of their N-aluminylene as they attempted to react the compound with various
In 2023, Liu and coworkers published further examples of the reactivity of their N-aluminylene as they attempted to react the compound with various
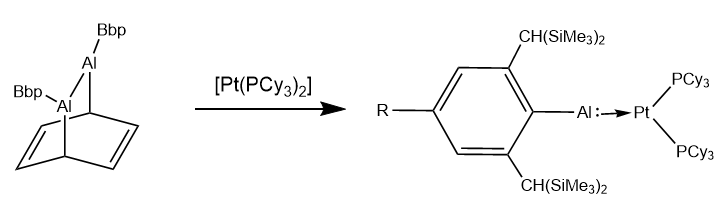 Aluminylenes have also demonstrated the ability to act as ligands and coordinate to
Aluminylenes have also demonstrated the ability to act as ligands and coordinate to  The N-aluminylene reported by Liu, also demonstrated an ability to coordinate to metal atoms. UV irradiation of
The N-aluminylene reported by Liu, also demonstrated an ability to coordinate to metal atoms. UV irradiation of
 Aluminylenes are a sub-class of
Aluminylenes are a sub-class of aluminium(I) compounds In chemistry, aluminium(I) refers to Monovalent ion, monovalent aluminium (+1 oxidation state) in both Ionic bonding, ionic and Covalent bond, covalent bonds. Along with aluminium(II), it is an extremely unstable form of aluminium.
While late Group ...
that feature singly-coordinated aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
atoms with a lone pair
In chemistry, a lone pair refers to a pair of valence electrons that are not shared with another atom in a covalent bondIUPAC ''Gold Book'' definition''lone (electron) pair''/ref> and is sometimes called an unshared pair or non-bonding pair. Lone ...
of electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s. As aluminylenes exhibit two unoccupied orbitals, they are not strictly aluminium analogues of carbene
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a Valence (chemistry), valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
Th ...
s until stabilized by a Lewis base
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
to form aluminium(I) nucleophiles. The lone pair and two empty orbitals on the aluminium allow for ambiphilic bonding where the aluminylene can act as both an electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively Electric charge, charged, have an ...
and a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
. Aluminylenes have also been reported under the names alumylenes and alanediyl.
The +1 oxidation state for aluminium is less stable than heavier group 13
The Group 13 network (, ) was a Jewish collaborationist organization in the Warsaw Ghetto during the German occupation of Poland in World War II. The rise and fall of the Group was likely a proxy for power struggles between various facti ...
elements, but the lower stability and higher reactivity of aluminium(I) compounds make for interesting chemistry. The first aluminium(I) compound to be isolated was Dohmeier's (AlCp*)4 which existed as a tetrameric solid but dissociated in solution to the monomer. This was followed by Roesky's synthesis of a doubly coordinated aluminium(I) and nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, proper ...
analogous to an aluminium Arduengo carbene. Despite some rich aluminium(I) chemistry following those discoveries, it wasn't until 2020 that a free (not Lewis base stabilized) aluminylene was synthesized.
Free aluminylenes
Simple aluminylenes have been studied but are highly reactive and only exist in the gas phase under extreme conditions. The first free aluminylene came from Tuononen and Power, who used bulky terphenylligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
s to stabilize the reduction of the aluminium(III) diiodide. The isolated arylaluminylene formed thermally stable yellow-orange crystals that were characterized via X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
and NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic f ...
. The aluminylene demonstrated more reactivity than its gallium
Gallium is a chemical element; it has Chemical symbol, symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875,
elemental gallium is a soft, silvery metal at standard temperature and pressure. ...
analogue and quickly formed an aluminium hydride upon reaction with hydrogen gas
Hydrogen is a chemical element; it has symbol H and atomic number 1. It is the lightest and most abundant chemical element in the universe, constituting about 75% of all normal matter. Under standard conditions, hydrogen is a gas of diatomi ...
.
Soon after, Liu and coworkers as well as Hinz and coworkers separately synthesized a free nitrogen bound aluminylenes that was stabilized with the use of bulky carbazolyl ligands. While also thermally stable, the N-aluminylene was extremely sensitive to air and water. Part of the stability of the N-aluminylene is based on slight pi-donation from the nitrogen atom, facilitated by the planar nature of the molecule. This conclusion is supported by electronic structure calculations and a slightly shorter N-Al bond distance than would be expected for a N-Al single bond. Both free aluminylenes largely depend on the steric bulk of their ligands for kinetic protection, a common motif in stabilizing reactive main group complexes.
Reactivity
The ambiphilic nature of aluminylenes, as well as the reactivity of aluminium(I) complexes more generally, allows for aluminylenes to participate in a diverse range of reactions.Natural Bond Orbital
In quantum chemistry, a natural bond orbital or NBO is a calculated ''bonding orbital'' with maximum electron density. The NBOs are one of a sequence of natural localized orbital sets that include "natural atomic orbitals" (NAO), "natural hybrid o ...
(NBO) calculations showed that the frontier orbitals of these aluminylenes matched expectations with the aluminium lone pair as the HOMO
''Homo'' () is a genus of great ape (family Hominidae) that emerged from the genus ''Australopithecus'' and encompasses only a single extant species, ''Homo sapiens'' (modern humans), along with a number of extinct species (collectively called ...
and a largely aluminium p-orbital based LUMO
In chemistry, HOMO and LUMO are types of molecular orbitals. The acronyms stand for ''highest occupied molecular orbital'' and ''lowest unoccupied molecular orbital'', respectively. HOMO and LUMO are sometimes collectively called the ''frontie ...
.
Redox reactions
 Power's aluminylene was shown to react with organic
Power's aluminylene was shown to react with organic azide
In chemistry, azide (, ) is a linear, polyatomic anion with the formula and structure . It is the conjugate base of hydrazoic acid . Organic azides are organic compounds with the formula , containing the azide functional group. The dominant ...
s to create aluminium(III) imides. In a reaction with ArMe6N3, the terphenyl aluminylene was able to form an Al-N triple bond, a conclusion supported by the shortest reported Al-N bond distances (1.625Å). This aluminylene also reacted with less bulky azides, but the lack of steric protection meant that a second equivalent of azide reacted to give a multiply coordinated aluminium(III) compound.
 The N-aluminylene reported by Liu and coworkers was shown to undergo an oxidative insertion reaction when mixed with IDippCuCl (IDipp=1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) to form a terminal
The N-aluminylene reported by Liu and coworkers was shown to undergo an oxidative insertion reaction when mixed with IDippCuCl (IDipp=1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene) to form a terminal copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
-alumanyl complex.
 Liu also demonstrated that the N-aluminylene could act as an important precursor to
Liu also demonstrated that the N-aluminylene could act as an important precursor to organoaluminium compounds
Organoaluminium chemistry is the study of compounds containing bonds between carbon and aluminium. It is one of the major themes within organometallic chemistry. Illustrative organoaluminium compounds are the dimer trimethylaluminium, the monomer ...
. In these reactions, the aluminylene performs cycloaddition
In organic chemistry, a cycloaddition is a chemical reaction in which "two or more Unsaturated hydrocarbon, unsaturated molecules (or parts of the same molecule) combine with the formation of a cyclic adduct in which there is a net reduction of th ...
with unsaturated hydrocarbons
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as α-olefins.
The International Union of Pu ...
to create aluminium heterocycles. Subsequently, the Al-N bond can be cleaved using a nucleophilic salt to free the newly formed organoaluminium compound.
 In 2023, Liu and coworkers published further examples of the reactivity of their N-aluminylene as they attempted to react the compound with various
In 2023, Liu and coworkers published further examples of the reactivity of their N-aluminylene as they attempted to react the compound with various boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
based Lewis acids. Upon reaction with Ph2BOBPh2, the aluminylene formed a tricoordinate species featuring new aluminium-boron and aluminium-oxygen bonds. This free alumaborane was characterized via 11B NMR and showed two three-coordinate boron atoms, an observation further supported by x-ray crystallography data. The formation of Lewis adduct
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any sp ...
s was also observed when the aluminylene was mixed with strong Lewis acids such as BCF (Tris(pentafluorophenyl)borane) and Piers’ borane (HB(C6F5)2).
Lewis base stabilized aluminylenes
In addition to free aluminylenes, there have been several attempts to further stabilize these reactive species through the coordination of another Lewis base. Transient versions of these compounds have been reported on the way to other products via coordination withN-heterocyclic Carbenes
A persistent carbene (also known as stable carbene) is an organic molecule whose natural resonance structure has a carbon atom with incomplete octet (a carbene), but does not exhibit the tremendous instability typically associated with such moiet ...
(NHCs) and amidophosphines. However, in 2022 Liu and coworkers were able to form an adduct between their N-aluminylene and an NHC, a combination that demonstrated increased reactivity compared to the free aluminylene. They explained this with Density Functional Theory
Density functional theory (DFT) is a computational quantum mechanical modelling method used in physics, chemistry and materials science to investigate the electronic structure (or nuclear structure) (principally the ground state) of many-body ...
calculations at the M06-2X/def2-SVP level showing that the NHC coordination narrowed of the HOMO-LUMO gap by raising the energy of the aluminium lone pair (HOMO). This aluminylene-NHC adduct was then shown to activate otherwise unreactive arene
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated."
The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were ...
species to initiate ring expansions.
Aluminylene coordination chemistry
 Aluminylenes have also demonstrated the ability to act as ligands and coordinate to
Aluminylenes have also demonstrated the ability to act as ligands and coordinate to transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
centers. Tokitoh demonstrated multiple methods for using dialumene starting materials to create an arylaluminylene platinum
Platinum is a chemical element; it has Symbol (chemistry), symbol Pt and atomic number 78. It is a density, dense, malleable, ductility, ductile, highly unreactive, precious metal, precious, silverish-white transition metal. Its name origina ...
complexes. NBO calculations showed that the Al-Pt bond showed a large degree of electrostatic interaction
Electrostatics is a branch of physics that studies slow-moving or stationary electric charges.
Since classical times, it has been known that some materials, such as amber, attract lightweight particles after rubbing. The Greek word (), meani ...
, supplemented by sigma donation from the aluminium and pi-backbonding from the platinum.
 The N-aluminylene reported by Liu, also demonstrated an ability to coordinate to metal atoms. UV irradiation of
The N-aluminylene reported by Liu, also demonstrated an ability to coordinate to metal atoms. UV irradiation of tungsten hexacarbonyl
Tungsten hexacarbonyl (also called tungsten carbonyl) is an organometallic compound with the formula W(CO)6. This complex gave rise to the first example of a dihydrogen complex.Kubas, G. J., Metal Dihydrogen and σ-Bond Complexes, Kluwer Academic ...
in the presence of the N-aluminylene created an aluminylene-W(CO)5 compound. Furthermore, treatment of the N-aluminylene with W(CO)6 and Cr(CO)6 in coordinating solvents such as THF
Tetrahydrofuran (THF), or oxolane, is an organic compound with the formula (CH2)4O. The compound is classified as heterocyclic compound, specifically a cyclic ether. It is a colorless, water-miscible organic liquid with low viscosity. It is ma ...
and DMAP also formed the aluminylene-transition metal complexes. In these cases, the aluminylene was stabilized by having a THF molecule or two DMAP molecules donate their lone pairs into the aluminylenes empty orbitals. Intrinsic Bond Orbital calculations showed a significant degree of pi-backbonding from the aluminylene in the tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
and chromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal.
Chromium ...
complexes, which added further stabilization.
References
{{Reflist Octet-deficient functional groups Aluminium(I) compounds Organoaluminium compounds Coordination complexes