|
Intrinsic Bond Orbitals
Intrinsic bond orbitals (IBO) are localized molecular orbitals giving exact and non-empirical representations of Wave function, wave functions. They are obtained by unitary transformation and form an orthogonal set of orbitals localized on a minimal number of atoms. IBOs present an intuitive and unbiased interpretation of chemical bonding with naturally arising Lewis structure, Lewis structures. For this reason IBOs have been successfully employed for the elucidation of molecular structures and electron flow along the intrinsic reaction coordinate (IRC). IBOs have also found application as Wannier function, Wannier functions in the study of solids. Theory The IBO method entails molecular wave-functions calculated using self-consistent field (SCF) methods such as Kohn-Sham density functional theory (DFT) which are expressed as linear combinations of localized molecular orbitals. In order to arrive at IBOs, intrinsic atomic orbitals (IAOs) are first calculated as representations of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
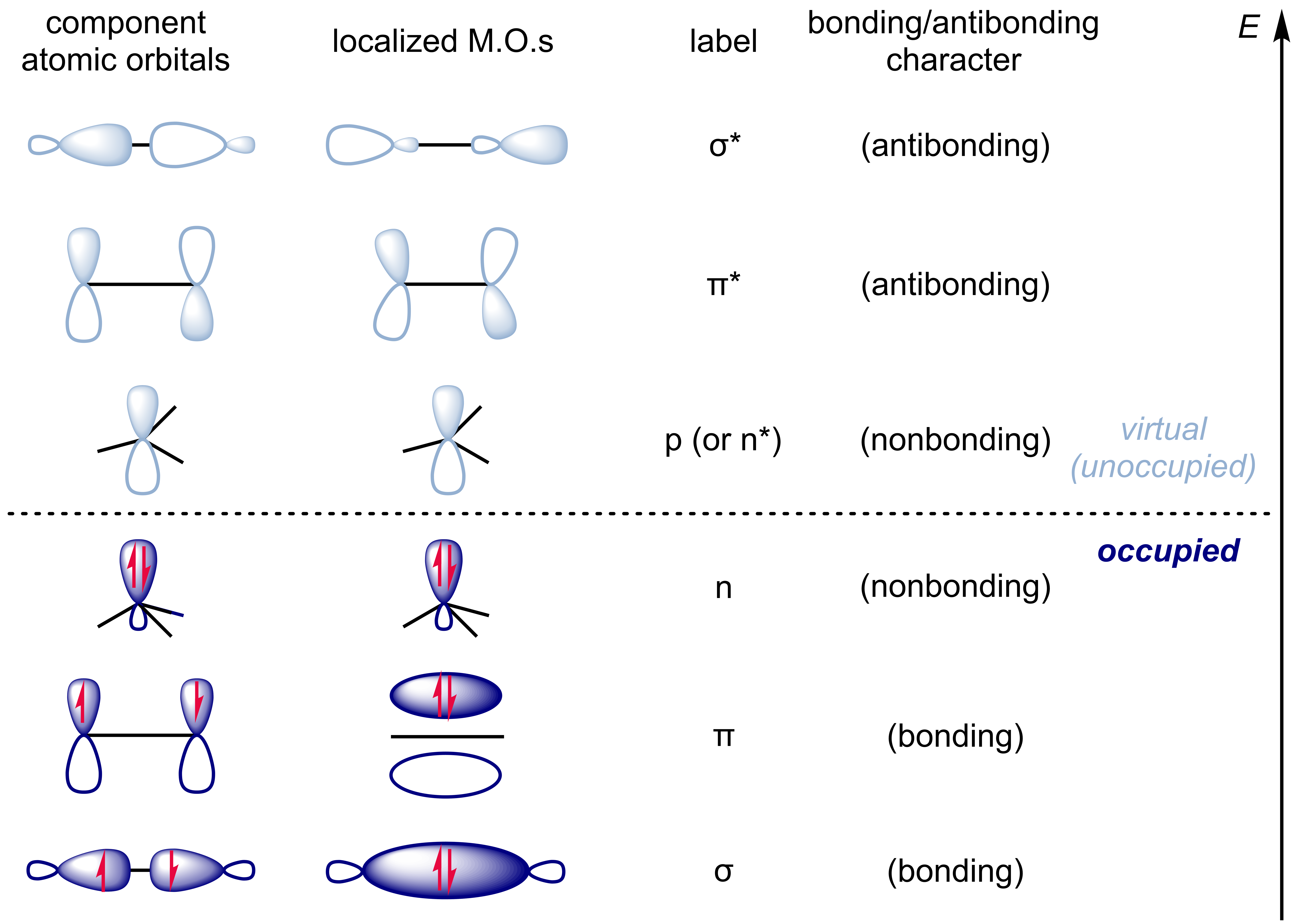
Localized Molecular Orbitals
Localized molecular orbitals are molecular orbitals which are concentrated in a limited spatial region of a molecule, such as a specific bond or lone pair on a specific atom. They can be used to relate molecular orbital calculations to simple bonding theories, and also to speed up post-Hartree–Fock electronic structure calculations by taking advantage of the local nature of electron correlation. Localized orbitals in systems with periodic boundary conditions are known as Wannier functions. Standard ab initio quantum chemistry methods lead to delocalized orbitals that, in general, extend over an entire molecule and have the symmetry of the molecule. Localized orbitals may then be found as linear combinations of the delocalized orbitals, given by an appropriate unitary transformation. In the water molecule for example, ab initio calculations show bonding character primarily in two molecular orbitals, each with electron density equally distributed among the two O-H bonds. The local ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
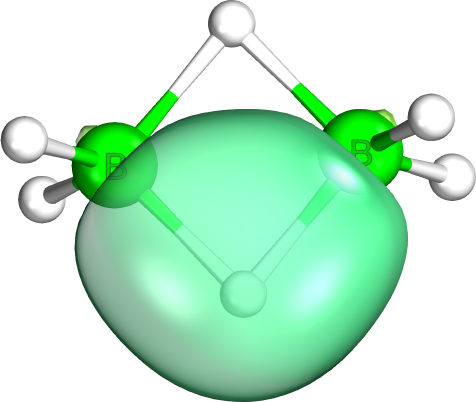
Three-center Two-electron Bond
A three-center two-electron (3c–2e) bond is an electron-deficient chemical bond where three atoms share two electrons. The combination of three atomic orbitals form three molecular orbitals: one bonding, one ''non''-bonding, and one ''anti''-bonding. The two electrons go into the bonding orbital, resulting in a net bonding effect and constituting a chemical bond among all three atoms. In many common bonds of this type, the bonding orbital is shifted towards two of the three atoms instead of being spread equally among all three. Example molecules with 3c–2e bonds are the trihydrogen cation () and diborane (). In these two structures, the three atoms in each 3c–2e bond form an angular geometry, leading to a bent bond. Boranes and carboranes An extended version of the 3c–2e bond model features heavily in cluster compounds described by the polyhedral skeletal electron pair theory, such as boranes and carboranes. These molecules derive their stability from having a com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lewis Acids And Bases
A Lewis acid (named for the American physical chemist Gilbert N. Lewis) is a chemical species that contains an empty orbital which is capable of accepting an electron pair from a Lewis base to form a Lewis adduct. A Lewis base, then, is any species that has a filled orbital containing an electron pair which is not involved in bonding but may form a dative bond with a Lewis acid to form a Lewis adduct. For example, NH3 is a Lewis base, because it can donate its lone pair of electrons. Trimethylborane CH3)3Bis a Lewis acid as it is capable of accepting a lone pair. In a Lewis adduct, the Lewis acid and base share an electron pair furnished by the Lewis base, forming a dative bond. In the context of a specific chemical reaction between NH3 and Me3B, a lone pair from NH3 will form a dative bond with the empty orbital of Me3B to form an adduct NH3•BMe3. The terminology refers to the contributions of Gilbert N. Lewis. From p. 142: "We are inclined to think of substances as po ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pentamethylcyclopentadiene
1,2,3,4,5-Pentamethylcyclopentadiene is a cyclic compound, cyclic diene with the formula , often written , where Me is Methyl group, . It is a colorless liquid. 1,2,3,4,5-Pentamethylcyclopentadiene is the precursor to the ligand ''1,2,3,4,5-pentamethylcyclopentadienyl'', which is often denoted Cp* () and read as "C P star", the "star" signifying the five methyl groups radiating from the core of the ligand. Thus, the 1,2,3,4,5-pentamethylcyclopentadiene's formula is also written Cp*H. In contrast to less-substituted cyclopentadiene derivatives, Cp*H is not prone to dimerization. Synthesis Pentamethylcyclopentadiene is commercially available. It was first prepared from tiglaldehyde and 2-butenyllithium, via 2,3,4,5-tetramethylcyclopent-2-enone, with a Nazarov cyclization reaction as a key step. : Alternatively, 2-butenyllithium adds to ethyl acetate followed by acid-catalyzed dehydrocyclization: : : Organometallic derivatives Cp*H is a precursor to organometallic compounds con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radical (chemistry)
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired valence electron. With some exceptions, these unpaired electrons make radicals highly chemically reactive. Many radicals spontaneously dimerize. Most organic radicals have short lifetimes. A notable example of a radical is the hydroxyl radical (HO·), a molecule that has one unpaired electron on the oxygen atom. Two other examples are triplet oxygen and triplet carbene (꞉) which have two unpaired electrons. Radicals may be generated in a number of ways, but typical methods involve redox reactions. Ionizing radiation, heat, electrical discharges, and electrolysis are known to produce radicals. Radicals are intermediates in many chemical reactions, more so than is apparent from the balanced equations. Radicals are important in combustion, atmospheric chemistry, polymerization, plasma chemistry, biochemistry, and many other chemical processes. A majority ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Distonic Ion
Distonic ions are Chemical substance, chemical species that contain ionic charges and radical sites in different locations (on separate atoms), unlike regular radicals where the formal charge and unpaired electron are in the same location. These molecular species are created by ionization of either zwitterions or diradicals; ultimately, a neutral molecule loses an electron. Through experimental research distonic radicals have been found to be extremely stable gas phase ions and can be separated into different classes depending on the inherent features of the charged portion of the ion. History In 1984 scientists Bouma, Radom and Yates originated the term through extensive experimental research but they were not the first to deal with distonic ions. Experiments date back to the 1970s with Gross and McLafferty who were the first to propose the idea of such a species. Ion structure Several efficient techniques are available to detect the presence of distonic ions; the most appro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rearrangement Reaction
In organic chemistry, a rearrangement reaction is a broad class of organic reactions where the carbon skeleton of a molecule is rearranged to give a structural isomer of the original molecule. Often a substituent moves from one atom to another atom in the same molecule, hence these reactions are usually intramolecular. In the example below, the substituent R moves from carbon atom 1 to carbon atom 2: :\underset\ce\ce\underset\ce\ce Intermolecular rearrangements also take place. A rearrangement is not well represented by simple and discrete electron transfers (represented by curved arrows in organic chemistry texts). The actual mechanism of alkyl groups moving, as in Wagner–Meerwein rearrangement, probably involves transfer of the moving alkyl group fluidly along a bond, not ionic bond-breaking and forming. In pericyclic reactions, explanation by orbital interactions give a better picture than simple discrete electron transfers. It is, nevertheless, possible to draw the curved ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vinylidene Group
In chemistry, vinylidenes are compounds with the functional group C=CH2. An example is 1,1-dichloroethene (CCl2=CH2) commonly called ''vinylidene chloride''. It and vinylidene fluoride are precursors to commercially useful polymers. Monomers and polymers Vinylidene chloride and fluoride can be converted to linear polymers polyvinylidene chloride (PVDC) and polyvinylidene fluoride (PVDF). The polymerization reaction is: : ''n'' CH2=CX2 → (CH2−CX2)''n'' These vinylidene polymers are isomeric with those produced from vinylene monomers. Thus polyvinylene fluoride from vinylene fluoride (HFC=CHF). Vinylidene complexes Although vinylidenes are only transient species, they are found as ligands in organometallic chemistry. They typically arise by the protonation of metal acetylides or by the reaction of metal electrophiles with terminal alkynes. The complex chloro(cyclopentadienyl)bis(triphenylphosphine)ruthenium readily forms such complexes: :CpRu(PPh3)2Cl + RC2H + K ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Au Carbene
Au, AU, au or a.u. may refer to: Science and technology Computing * .au, the internet country code for Australia * Au file format, Sun Microsystems' audio format * Audio Units, a system level plug-in architecture from Apple Computer * Adobe Audition, a sound editor program * Windows Update or Automatic Updates, in Microsoft Windows * Windows 10 Anniversary Update, of August 2016a * Gold, chemical symbol Au * Absorbance unit, a reporting unit in spectroscopy * Atomic units, a system of units convenient for atomic physics and other fields * Ångström unit, a unit of length equal to 10−10 m or 0.1 nanometre. * Astronomical unit, a unit of length used in planetary systems astronomy * Arbitrary unit, a placeholder unit for when the actual value of a measurement is unknown or unimportant Arts and entertainment Music * AU (band), an experimental pop group headed by Luke Wyland * ''Au'', a 2010 release by Scottish rock band Donaldson, Moir and Paterson * ''Au'' a track on Some Time ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coinage Metal N-Heterocyclic Carbene (NHC) Complexes
Coinage metal ''N''-heterocyclic carbene (NHC) complexes refer to transition metal complexes incorporating at least one Group 11 element, coinage metal center (M = Cu, Ag, Au) Chemical ligation, ligated by at least one NHC-type persistent carbene. A variety of such complexes have been synthesized through deprotonation of the appropriate Imidazolium#Salts of imidazole, imidazolium precursor and metalation by the appropriate metal source, producing MI, MII, or MIII NHC complexes. While the general form can be represented as (R2N)2C:–M (R = various alkyl or aryl groups), the exact nature of the bond between NHC and M has been investigated extensively through computational modeling and experimental probes. These results indicate that the M-NHC bond consists mostly of Non-covalent interaction#Electrostatic interactions, electrostatic attractive interactions, with some covalent bond character arising from NHC to M σ donation and minor M to NHC Pi backbonding, π back-donation. Coinage me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |