aluminium(I) on:
[Wikipedia]
[Google]
[Amazon]
In chemistry, aluminium(I) refers to monovalent Al^3+ ) is the much more common oxidation state for aluminium.
Aluminium(I) compounds are both prone to
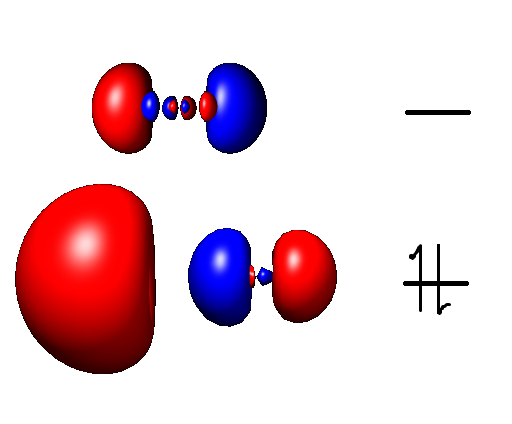 Al(I) appears to be red, as solutions of AlBr and AlCl in organic solvents are both red. The presence of this color implies a relatively small HOMO/LUMO gap that is accessible by green light.
The geometry of compounds can be determined by analysis of the fine structure of the electronic spectra. Matrix isolation spectroscopy prevents disproportionation of aluminium monohalides and thus allows for the measuring of transitional vibrations as well as reactivity with molecules such as O2.
Analysis by 27Al
Al(I) appears to be red, as solutions of AlBr and AlCl in organic solvents are both red. The presence of this color implies a relatively small HOMO/LUMO gap that is accessible by green light.
The geometry of compounds can be determined by analysis of the fine structure of the electronic spectra. Matrix isolation spectroscopy prevents disproportionation of aluminium monohalides and thus allows for the measuring of transitional vibrations as well as reactivity with molecules such as O2.
Analysis by 27Al
Al (l) + HCl (g) -> AlCl (g) + 1/2 H2 (g)
Due to the nature of HF, which possesses a bond much stronger than that of its congeners, AlF is synthesized instead by the 2 Al (s) + AlF3 (s) -> 3 AlF (g)
Stability increases with mass: while AlCl decomposes at 77 K or above, AlBr remains stable up to 253 K. Remarkably, it has been discovered that at any given temperature, the vapor pressure of AlF is orders of magnitude lower than that of other aluminium monohalides.
2 AlCl (s)-> Al (s) +AlCl3 (s)
Dohmier et al. documented that the exception is AlBr. AlBr is stable enough at temperatures under -30 C that it comproportionates to AlBr2 in the presence of AlBr3.
:AlBr (s) + AlBr3 (s) -> 2 AlBr2 (s)
 In 2018, Liu et al. reviewed the chemistry of aluminium (I) with β-diketiminato ligands, widely used ligands with immense versatility in electronic and steric properties. These aluminium (I) complexes have immense potential for small molecule activation.
Synthesis
β-diketiminato aluminium alkyls and aluminium halides are synthesized by adding a trialkyl aluminium compound to the initial β-diketiminate ligands, adding iodine, and the reducing with potassium.
In 2018, Liu et al. reviewed the chemistry of aluminium (I) with β-diketiminato ligands, widely used ligands with immense versatility in electronic and steric properties. These aluminium (I) complexes have immense potential for small molecule activation.
Synthesis
β-diketiminato aluminium alkyls and aluminium halides are synthesized by adding a trialkyl aluminium compound to the initial β-diketiminate ligands, adding iodine, and the reducing with potassium.

 This method of cluster formation created the only known incidence of an octahedral aluminium cluster, l6(tBu)6sup>−, which was formed by reaction between AlCl and tBuLi. Similarly, AlCl and LiN(SiMe3)2 react to form the first known example of a cluster where two M4 tetrahedra are connected by a common center.
This method of cluster formation created the only known incidence of an octahedral aluminium cluster, l6(tBu)6sup>−, which was formed by reaction between AlCl and tBuLi. Similarly, AlCl and LiN(SiMe3)2 react to form the first known example of a cluster where two M4 tetrahedra are connected by a common center.
aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
(+1 oxidation state) in both ionic and covalent
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs. The stable balance of attractive and repulsive forces between atom ...
bonds. Along with aluminium(II), it is an extremely unstable form of aluminium.
While late Group 13 element
The Group 13 network (, ) was a Jewish collaborationist organization in the Warsaw Ghetto during the German occupation of Poland in World War II. The rise and fall of the Group was likely a proxy for power struggles between various facti ...
s such as thallium
Thallium is a chemical element; it has Symbol (chemistry), symbol Tl and atomic number 81. It is a silvery-white post-transition metal that is not found free in nature. When isolated, thallium resembles tin, but discolors when exposed to air. Che ...
and indium
Indium is a chemical element; it has Symbol (chemistry), symbol In and atomic number 49. It is a silvery-white post-transition metal and one of the softest elements. Chemically, indium is similar to gallium and thallium, and its properties are la ...
prefer the +1 oxidation state, aluminium(I) is rare. Aluminium does not experience the inert-pair effect, a phenomenon where valence s electrons are poorly shielded from nuclear charge due to the presence of filled d and f orbitals. As such, aluminium (III) (disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionatio ...
and difficult to prepare. At standard conditions, they readily oxidize to the aluminium(III) form.
Characteristics
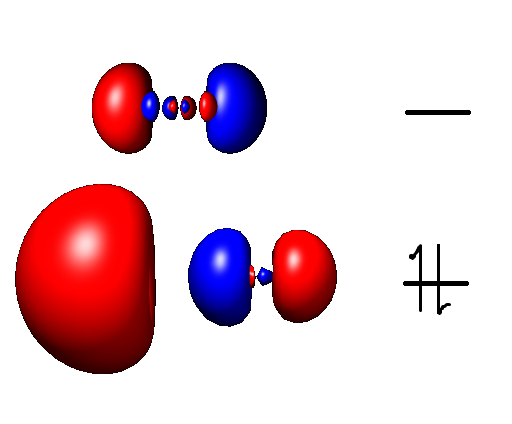 Al(I) appears to be red, as solutions of AlBr and AlCl in organic solvents are both red. The presence of this color implies a relatively small HOMO/LUMO gap that is accessible by green light.
The geometry of compounds can be determined by analysis of the fine structure of the electronic spectra. Matrix isolation spectroscopy prevents disproportionation of aluminium monohalides and thus allows for the measuring of transitional vibrations as well as reactivity with molecules such as O2.
Analysis by 27Al
Al(I) appears to be red, as solutions of AlBr and AlCl in organic solvents are both red. The presence of this color implies a relatively small HOMO/LUMO gap that is accessible by green light.
The geometry of compounds can be determined by analysis of the fine structure of the electronic spectra. Matrix isolation spectroscopy prevents disproportionation of aluminium monohalides and thus allows for the measuring of transitional vibrations as well as reactivity with molecules such as O2.
Analysis by 27Al NMR spectroscopy
Nuclear magnetic resonance spectroscopy, most commonly known as NMR spectroscopy or magnetic resonance spectroscopy (MRS), is a spectroscopic technique based on re-orientation of atomic nuclei with non-zero nuclear spins in an external magnetic f ...
of AlCl, AlBr, and AlI
Ali ibn Abi Talib (; ) was the fourth Rashidun caliph who ruled from until his assassination in 661, as well as the first Shia Imam. He was the cousin and son-in-law of the Islamic prophet Muhammad. Born to Abu Talib ibn Abd al-Muttalib an ...
in toluene/diethyl ether at room temperature reveal two signals: one very broad signal at δ = 100-130 ppm (regardless of the halogen), and one at higher field strength (AlCl: δ = + 30, AlBr: δ = + 50, AlI: δ = + 80). The first signal corresponds to a donor-stabilized four-coordinate aluminium species, while the identity of the latter is inconclusive.
Monohalides
The aluminium(I) cation reacts with hydrogen halides to form the following aluminium monohalides: * aluminium monofluoride (AlF) * aluminium monochloride (AlCl) * aluminium monobromide (AlBr) * aluminium monoiodide (AlI) These compounds are only thermodynamically stable at high temperatures and low pressures in the singlet ground state. However, decomposition can be prevented by making disproportionation kinetically unfavorable. Under cold temperatures (below 77 K), disproportionation is slow enough that the AlCl solid can be kept for long periods of time.Synthesis
AlCl is synthesized by reaction of liquid aluminium with gaseous HCl at 1200 K and 0.2 mbar to yield gaseous AlCl and hydrogen gas. At 77 K, AlCl is a dark red solid which turns black upon disproportionation at temperatures higher than 180 K. At temperatures under 77 K and dissolved in a matrix of polar and non-polar solvents, it exists as a metastable solution whose reactivity can be studied. AlBr, a red oil, is prepared similarly from liquid aluminium and gaseous HBr. :comproportionation
Comproportionation or symproportionation is a chemical reaction where two reactants containing the same element but with different oxidation numbers, form a compound having an intermediate oxidation number. It is the opposite of disproportionatio ...
of Al and AlF3 which are pressed and mixed into pellets. The pellets are then loaded into a graphite furnace and heated to 1050 K.
:Disproportionation
At room temperature, AlX compounds tend to disproportionate to Al and AlX3. When dark red, solid AlCl is allowed to warm up, it turns black to yield aluminium and the more stable aluminium (III) chloride salt. :Oligomerization
In Lewis basic solutions, AlX compounds have a tendency to oligomerize.
Complex chemistry
Aluminium is not only the most abundant metal in the Earth's crust, but also an element of low toxicity. As such, aluminium (I) complexes attract considerable interest. These complexes can be supported by various ligands and used to activate small molecules.β-Diketiminato systems
 In 2018, Liu et al. reviewed the chemistry of aluminium (I) with β-diketiminato ligands, widely used ligands with immense versatility in electronic and steric properties. These aluminium (I) complexes have immense potential for small molecule activation.
Synthesis
β-diketiminato aluminium alkyls and aluminium halides are synthesized by adding a trialkyl aluminium compound to the initial β-diketiminate ligands, adding iodine, and the reducing with potassium.
In 2018, Liu et al. reviewed the chemistry of aluminium (I) with β-diketiminato ligands, widely used ligands with immense versatility in electronic and steric properties. These aluminium (I) complexes have immense potential for small molecule activation.
Synthesis
β-diketiminato aluminium alkyls and aluminium halides are synthesized by adding a trialkyl aluminium compound to the initial β-diketiminate ligands, adding iodine, and the reducing with potassium.
+2Cycloadditions
Al(I) compounds exhibit behavior analogous to that of singletcarbenes
In organic chemistry, a carbene is a molecule containing a neutral carbon atom with a valence of two and two unshared valence electrons. The general formula is or where the R represents substituents or hydrogen atoms.
The term "carbene" may ...
. Like carbenes, they undergo +2cycloadditions with alkynes and azides to afford three membered ring derivatives such as dialuminacyclohexadiene.
Similarly to the nucleophilic carbon center in the carbene, the lone pair on the aluminium center binds to the first azide equivalent. Nitrogen gas is liberated. With the second equivalent of azide, a five-member ring is formed.
Reactions with other small molecules
Such aluminium (I) complexes can activate water as well as elemental phosphorus, oxygen, and sulfur to yield bridged dimers. This occurs via partial reduction of the elemental small molecule.AlCp*
AlCp*, consisting of aluminium (I) bonded with the pentamethylcyclopentadiene anion ((CCH3)5−), was first synthesized in 1991 by Dohmier et al. (AlCp*)4, a yellow crystalline solid, is first produced from the combination of AlCl and MgCp*2. When vaporized, the long Al-Al bonds (276.9 pm) split, and monomeric molecules of lCp*are created. As revealed through Schnockel's work, lCp*reacts by inserting itself into other bonds. Reaction with Al2I6 yields subvalent halide species; reaction with As4tBu4 yields As-Al bonds. When reacted with transition metal-cyclopentadienyl complexes such as NiCp2, it offers a straightforward pathway to compounds containing aluminium-transition metal bonds, which has great potential for important catalytic reactions. As with other AlR ligands, lCp*can be regarded as a CO analogue, as it possesses 2 empty π orbitals and engages in similar coordination modes (terminal and bridging). This similarity implies the possibility ofpi backbonding
In chemistry, pi backbonding or π backbonding is a π-bonding interaction between a filled (or half filled) orbital of a transition metal atom and a vacant orbital on an adjacent ion or molecule. In this type of interaction, electrons from the ...
interactions between AlCp*and metals it complexes to.
Metalloidal clusters
Work in aluminium clusters has been done by Linti and Schnockel. These metalloidal clusters can be formed from Al(I) compounds, namely aluminium monohalides. These clusters are termed "metalloidal clusters" because the number of unbridged metal-metal bonds is greater than the number of localized metal-ligand bonds. On the way to metal formation, intermediates are trapped in the presence of the bulky ligands which substitute the halide atoms. As a result, metal-rich clusters such as Al77R20 are possible and offer insight into solid bulk metal formation. Tetrahedral aluminium is available from the reaction between aluminium(I) species and organometallic species. These clusters can be made through combinations such as AlCp* and LiR, AlBr and Li(THF)3(SiMe3)3, and AlI and NaSiBu3.
 This method of cluster formation created the only known incidence of an octahedral aluminium cluster, l6(tBu)6sup>−, which was formed by reaction between AlCl and tBuLi. Similarly, AlCl and LiN(SiMe3)2 react to form the first known example of a cluster where two M4 tetrahedra are connected by a common center.
This method of cluster formation created the only known incidence of an octahedral aluminium cluster, l6(tBu)6sup>−, which was formed by reaction between AlCl and tBuLi. Similarly, AlCl and LiN(SiMe3)2 react to form the first known example of a cluster where two M4 tetrahedra are connected by a common center.
Natural occurrence
Aluminium is rarely found in its +1 oxidation state in nature due to the immense stability of the +3 oxidation state. Rotational transitions of AlF and AlCl have been detected in circumstellar shells nearIRC +10216
CW Leonis or IRC +10216 is a variable carbon star that is embedded in a thick dust envelope. It was first discovered in 1969 by a group of astronomers led by Eric Becklin, based upon infrared observations made with the 62-inch Caltech ...
. The presence of AlF suggests that fluorine is produced in helium shell flashes instead of explosive nucleosynthesis.
See also
* AluminyleneReferences