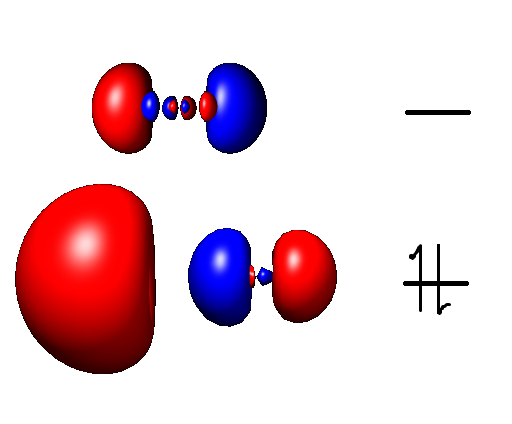|
Aluminium Monofluoride
Aluminium monofluoride, also known as fluoridoaluminium, is the chemical compound with the formula AlF. This elusive species is formed by the reaction between aluminium trifluoride and metallic aluminium at elevated temperatures but quickly reverts to the reactants when cooled. Clusters derived from related aluminium(I) halides can be stabilized using specialized ligands. This molecule has been detected in the interstellar medium The interstellar medium (ISM) is the matter and radiation that exists in the outer space, space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as cosmic dust, dust and cosmic rays. It f ..., where molecules are so dilute that intermolecular collisions are unimportant. See also * Aluminium monobromide * Aluminium monochloride * Aluminium monoiodide References Aluminium(I) compounds Fluorides Metal halides Diatomic molecules {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Monochloride
Aluminium monochloride, or chloridoaluminium is the metal halide with the formula AlCl. Aluminium monochloride as a molecule is thermodynamically stable at high temperature and low pressure only. This compound is produced as a step in the Alcan process to smelt aluminium from an aluminium-rich alloy. When the alloy is placed in a reactor that is heated to 1,300 °C and mixed with aluminium trichloride, a gas of aluminium monochloride is produced. : It then disproportionates into aluminium melt and aluminium trichloride upon cooling to 900 °C. This molecule has been detected in the interstellar medium The interstellar medium (ISM) is the matter and radiation that exists in the outer space, space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as cosmic dust, dust and cosmic rays. It f ..., where molecules are so dilute that intermolecular collisions are unimportant. See also * Aluminium monofluoride * A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis acids and bases, Lewis bases. The nature of metal–ligand bonding can range from covalent bond, covalent to ionic bond, ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acids and bases, Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity (chemistry), reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorides
Fluoride (). According to this source, is a possible pronunciation in British English. is an inorganic, monatomic anion of fluorine, with the chemical formula (also written ), whose salts are typically white or colorless. Fluoride salts typically have distinctive bitter tastes, and are odorless. Its salts and minerals are important chemical reagents and industrial chemicals, mainly used in the production of hydrogen fluoride for fluorocarbons. Fluoride is classified as a weak base since it only partially associates in solution, but concentrated fluoride is corrosive and can attack the skin. Fluoride is the simplest fluorine anion. In terms of charge and size, the fluoride ion resembles the hydroxide ion. Fluoride ions occur on Earth in several minerals, particularly fluorite, but are present only in trace quantities in bodies of water in nature. Nomenclature Fluorides include compounds that contain ionic fluoride and those in which fluoride does not dissociate. The nome ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium(I) Compounds
In chemistry, aluminium(I) refers to Monovalent ion, monovalent aluminium (+1 oxidation state) in both Ionic bonding, ionic and Covalent bond, covalent bonds. Along with aluminium(II), it is an extremely unstable form of aluminium. While late Group 13 elements such as thallium and indium prefer the +1 oxidation state, aluminium(I) is rare. Aluminium does not experience the inert-pair effect, a phenomenon where valence s electrons are poorly shielded from nuclear charge due to the presence of filled d and f orbitals. As such, aluminium (III) (Al^3+) is the much more common oxidation state for aluminium. Aluminium(I) compounds are both prone to disproportionation and difficult to prepare. At standard conditions, they readily oxidize to the aluminium(III) form. Characteristics Al(I) appears to be red, as solutions of Aluminium monobromide, AlBr and Aluminium monochloride, AlCl in organic solvents are both red. The presence of this color implies a relatively small HOMO/LUMO gap that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Monoiodide
Aluminium monoiodide is an aluminium(I) compound with the chemical formula . It is unstable at room temperature due to dismutation: : It forms a cyclic adduct with triethylamine Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor remini .... See also * Aluminium monofluoride * Aluminium monochloride * Aluminium monobromide References Aluminium(I) compounds Iodides Metal halides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Monochloride
Aluminium monochloride, or chloridoaluminium is the metal halide with the formula AlCl. Aluminium monochloride as a molecule is thermodynamically stable at high temperature and low pressure only. This compound is produced as a step in the Alcan process to smelt aluminium from an aluminium-rich alloy. When the alloy is placed in a reactor that is heated to 1,300 °C and mixed with aluminium trichloride, a gas of aluminium monochloride is produced. : It then disproportionates into aluminium melt and aluminium trichloride upon cooling to 900 °C. This molecule has been detected in the interstellar medium The interstellar medium (ISM) is the matter and radiation that exists in the outer space, space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as cosmic dust, dust and cosmic rays. It f ..., where molecules are so dilute that intermolecular collisions are unimportant. See also * Aluminium monofluoride * A ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aluminium Monobromide
Aluminium monobromide is a chemical compound with the empirical formula In chemistry, the empirical formula of a chemical compound is the simplest whole number ratio of atoms present in a compound. A simple example of this concept is that the empirical formula of sulfur monoxide, or SO, is simply SO, as is the empir ... AlBr. It forms from the reaction of HBr with Al metal at high temperature. It disproportionates near room temperature: :6/n " lBrsub>n" → Al2Br6 + 4 Al This reaction is reversed at temperatures higher than 1000 °C. A more stable compound of aluminium and bromine is aluminium tribromide. See also * Aluminium monofluoride * Aluminium monochloride * Aluminium monoiodide External links Aluminium monobromide NIST Standard Reference Data Program Aluminium(I) compounds Bromides {{inorganic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Collision Theory
Collision theory is a principle of chemistry used to predict the rates of chemical reactions. It states that when suitable particles of the Reagent, reactant hit each other with the correct orientation, only a certain amount of collisions result in a perceptible or notable change; these successful changes are called successful collisions. The successful collisions must have enough energy, also known as activation energy, at the moment of impact to break the pre-existing bonds and form all new bonds. This results in the products of the reaction. The activation energy is often predicted using the transition state theory. Increasing the concentration of the reactant brings about more collisions and hence more successful collisions. Increasing the temperature increases the average kinetic energy of the molecules in a solution, increasing the number of collisions that have enough energy. Collision theory was proposed independently by Max Trautz in 1916 and William Lewis (physical chemis ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interstellar Medium
The interstellar medium (ISM) is the matter and radiation that exists in the outer space, space between the star systems in a galaxy. This matter includes gas in ionic, atomic, and molecular form, as well as cosmic dust, dust and cosmic rays. It fills interstellar space and blends smoothly into the surrounding intergalactic medium. The energy that occupies the same volume, in the form of electromagnetic radiation, is the interstellar radiation field. Although the density of atoms in the ISM is usually far below that in the best laboratory vacuums, the mean free path between collisions is short compared to typical interstellar lengths, so on these scales the ISM behaves as a gas (more precisely, as a Plasma (physics), plasma: it is everywhere at least slightly ionized), responding to pressure forces, and not as a collection of non-interacting particles. The interstellar medium is composed of multiple phases distinguished by whether matter is ionic, atomic, or molecular, and the temp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halides
In chemistry, a halide (rarely halogenide) is a binary chemical compound, of which one part is a halogen atom and the other part is an element or radical that is less electronegative (or more electropositive) than the halogen, to make a fluoride, chloride, bromide, iodide, astatide, or theoretically tennesside compound. The alkali metals combine directly with halogens under appropriate conditions forming halides of the general formula, MX (X = F, Cl, Br or I). Many salts are halides; the ''hal-'' syllable in ''halide'' and ''halite'' reflects this correlation. A halide ion is a halogen atom bearing a negative charge. The common halide anions are fluoride (), chloride (), bromide (), and iodide (). Such ions are present in many ionic halide salts. Halide minerals contain halides. All these halide anions are colorless. Halides also form covalent bonds, examples being colorless TiF4, colorless TiCl4, orange TiBr4, and brown TiI4. The heavier members TiCl4, TiBr4, TiI4 c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lithium Fluoride
Lithium fluoride is an inorganic compound with the chemical formula LiF. It is a colorless solid that transitions to white with decreasing crystal size. Its structure is analogous to that of sodium chloride, but it is much less soluble in water. It is mainly used as a component of molten salts. Partly because Li and F are both light elements, and partly because is highly reactive, formation of LiF from the elements releases one of the highest energies per mass of reactants, second only to that of BeO. Manufacturing LiF is prepared from lithium hydroxide or lithium carbonate with hydrogen fluoride. Applications Precursor to lithium hexafluorophosphate for batteries Lithium fluoride is reacted with hydrogen fluoride (HF) and phosphorus pentachloride to make lithium hexafluorophosphate , an ingredient in lithium ion battery electrolyte. The lithium fluoride alone does not absorb hydrogen fluoride to form a bifluoride salt. In molten salts Fluorine is produced by the elec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cluster Chemistry
Nanoclusters are atomically precise, crystalline materials most often existing on the 0-2 nanometer scale. They are often considered kinetically stable intermediates that form during the synthesis of comparatively larger materials such as semiconductor and metallic nanocrystals. The majority of research conducted to study nanoclusters has focused on characterizing their crystal structures and understanding their role in the nucleation and growth mechanisms of larger materials. Materials can be categorized into three different regimes, namely bulk, nanoparticles and nanoclusters. Bulk metals are electrical conductors and good optical reflectors and metal nanoparticles display intense colors due to surface plasmon resonance. However, when the size of metal nanoclusters is further reduced to form a nanocluster, the band structure becomes discontinuous and breaks down into discrete energy levels, somewhat similar to the energy levels of molecules. This gives nanoclusters similar q ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |