Aldonic Acid on:
[Wikipedia]
[Google]
[Amazon]
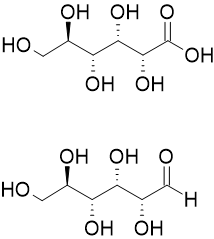 Aldonic acids are
Aldonic acids are

 Aldonic acids are
Aldonic acids are sugar acids
In organic chemistry, a sugar acid or acidic sugar is a monosaccharide with a carboxyl group at one end or both ends of its chain.
Main classes of sugar acids include:
* Aldonic acids, in which the aldehyde group () located at the initial end ( ...
with the general chemical formula, HO2C(CHOH)nCH2OH. They are obtained by oxidizing the aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
(-CHO group) of an aldose
An aldose is a monosaccharide (a simple sugar) with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms. Aldoses can be distinguished from ket ...
to form a carboxylic acid
In organic chemistry, a carboxylic acid is an organic acid that contains a carboxyl group () attached to an Substituent, R-group. The general formula of a carboxylic acid is often written as or , sometimes as with R referring to an organyl ...
(-COOH group). Aldonic acids are generally found in their ring form. However, these rings do not have a chiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
carbon at the terminal end bearing the aldehyde, and they cannot form R−O−R′ linkages between different molecules.
The nomenclature of aldonic acids and their lactones is based on replacing the suffix "-ose" with "onic acid" or "onolactone". Hence, D-glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ...
is oxidized to D-gluconic acid
Gluconic acid is an organic compound with molecular formula C6H12O7 and condensed structural formula HOCH2(CHOH)4CO2H. A white solid, it forms the gluconate anion in neutral aqueous solution. The salts of gluconic acid are known as "gluconates" ...
and D- gluconolactone.
Inventory
Sugar acids are white, water-soluble solids. They tend to dehydrate to the lactone derivative, often before they can be melted. All are chiral and, at least in nature, enantiopure.Synthesis
Oxidation by bromine and water
Aldonic acids are most commonly prepared by the oxidation of the sugar withbromine
Bromine is a chemical element; it has chemical symbol, symbol Br and atomic number 35. It is a volatile red-brown liquid at room temperature that evaporates readily to form a similarly coloured vapour. Its properties are intermediate between th ...
and water under neutral pH.

Strecker reaction
Alternatively, they arise by homologation of an aldose using theStrecker reaction Strecker is a German surname. Notable people with the surname include:
* Adolph Strecker (1822–1871), German chemist who worked with amino acids
* Herman Strecker (1836–1901), American entomologist specialising in butterflies and moths
* Heinri ...
. Cyanide in ammonia reacts with an aldose to produce an intermediate, which is then reacted with a hydronium ion to form an aldonic acid.
Oxidation by Benedict's and Fehling's reagents
Aldonic acids are the products of the oxidation ofaldoses
An aldose is a monosaccharide (a simple sugar) with a carbon backbone chain with a carbonyl group on the endmost carbon atom, making it an aldehyde, and hydroxyl groups connected to all the other carbon atoms. Aldoses can be distinguished from ket ...
by Benedict's or Fehling's reagents. Copper ions react with an aldose to form a red precipitate, Cu2O.
Natural synthesis
Anaerobic bacteria can also perform dehydrogenation to produce aldonic acids. This is done by synthesizing enzymes that are able to selectively oxidize aldoses to their corresponding aldonic acid.Applications
In commercial settings,glucose
Glucose is a sugar with the Chemical formula#Molecular formula, molecular formula , which is often abbreviated as Glc. It is overall the most abundant monosaccharide, a subcategory of carbohydrates. It is mainly made by plants and most algae d ...
, galactose
Galactose (, ''wikt:galacto-, galacto-'' + ''wikt:-ose#Suffix 2, -ose'', ), sometimes abbreviated Gal, is a monosaccharide sugar that is about as sweetness, sweet as glucose, and about 65% as sweet as sucrose. It is an aldohexose and a C-4 epime ...
, or arabinose
Arabinose is an aldopentose – a monosaccharide containing five carbon atoms, and including an aldehyde (CHO) functional group.
Properties
For biosynthetic reasons, most saccharides are almost always more abundant in nature as the "D"-form, o ...
are commonly oxidized to obtain aldonic acids. These products can then be used as the building blocks for preservatives, buffering agents, and other chemicals. As such, the use of aldonic acids for chemical applications is of growing interest to various industries.
Aldonic acids can be used as the natural starting materials to synthetic products including polyesters
Polyester is a category of polymers that contain one or two ester linkages in every repeat unit of their main chain. As a specific material, it most commonly refers to a type called polyethylene terephthalate (PET). Polyesters include some natura ...
and polyurethane
Polyurethane (; often abbreviated PUR and PU) is a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane term ...
. The incorporation of these organic sugars into synthetic materials allow for a more renewable alternative to oil-based polymer synthesis, and increased structural durability within polymer chains.
Properties
Aldonic acids are typically used in industrial applications for their ability to degrade naturally in the environment. This can be attributed to their affinity with water, as the polar bonds within the carboxylic acid group of aldonic acids allow them to interact with aquatic systems. The structural diversity of aldonic acids also allow for various properties. Their ring formation creates an added layer of rigidity when integrated with other materials.See also
* Aldaric acids * Uronic acidsReferences
{{reflist Sugar acids