|
Yellowcake Uranium
Yellowcake (also called urania) is a type of powdered uranium concentrate obtained from leach solutions, in an intermediate step in the processing of uranium ores. It is a step in the processing of uranium after it has been mined but before fuel fabrication or uranium enrichment. Yellowcake concentrates are prepared by various extraction and refining methods, depending on the types of ores. Typically, yellowcakes are obtained through the milling and chemical processing of uranium ore, forming a coarse powder that has a pungent odor, is insoluble in water, and contains about 80% uranium oxide, which melts at approximately 2880 °C. Overview Originally, raw uranium ore was extracted by traditional mining, and this is still the case in many mines. It is first crushed to a fine powder by passing it through crushers and grinders to produce "pulped" ore. This is further processed with concentrated acid, alkaline, or peroxide solutions to leach out the uranium. However, nearl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Yellowcake (03010301)
Yellowcake (also called urania) is a type of powder (substance), powdered uranium concentrate obtained from In-situ leach, leach solutions, in an intermediate step in the processing of uranium ores. It is a step in the processing of uranium after it has been mined but before fuel fabrication or uranium enrichment. Yellowcake concentrates are prepared by various extraction and refining methods, depending on the types of ores. Typically, yellowcakes are obtained through the milling and chemical processing of uranium ore, forming a coarse powder that has a pungent odor, is insoluble in water, and contains about 80% uranium oxide, which melts at approximately 2880 °C. Overview Originally, raw uranium ore was extracted by traditional mining, and this is still the case in many mines. It is first crushed to a fine powder by mineral processing, passing it through crushers and grinders to produce "pulped" ore. This is further processed with concentrated acid, alkaline, or peroxide ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Uranyl Peroxide
Uranyl peroxide or uranium peroxide hydrate (UO4·nH2O) is a pale-yellow, soluble peroxide of uranium. It is found to be present at one stage of the enriched uranium fuel cycle and in yellowcake prepared via the ''in situ'' leaching and resin ion exchange system. This compound, also expressed as UO3·(H2O2)·(H2O), is very similar to uranium trioxide hydrate UO3·''n''H2O. The dissolution behaviour of both compounds are very sensitive to the hydration state (n can vary between 0 and 4). One main characteristic of uranium peroxide is that it consists of small needles with an average AMAD of about 1.1 μm. The uranyl minerals studtite, UO4·4H2O, and metastudtite, UO4·2H2O, are the only minerals discovered to date found to contain peroxide. The product is a light yellow powder. Synthesis In general, uranyl peroxide can be obtained from a solution of uranium(VI) by adding a peroxide, usually hydrogen peroxide solution. The dihydrate is obtained from a boiling solution of urany ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gas Centrifuge
A gas centrifuge is a device that performs isotope separation of gases. A centrifuge relies on the principles of centrifugal force accelerating molecules so that particles of different masses are physically separated in a gradient along the radius of a rotating container. A prominent use of gas centrifuges is for the separation of uranium-235 (235U) from uranium-238 (238U). The gas centrifuge was developed to replace the gaseous diffusion method of 235U extraction. High degrees of separation of these isotopes relies on using many individual centrifuges arranged in series that achieve successively higher concentrations. This process yields higher concentrations of 235U while using significantly less energy compared to the gaseous diffusion process. History Suggested in 1919, the centrifugal process was first successfully performed in 1934. American scientist Jesse Beams and his team at the University of Virginia developed the process by separating two isotopes of chlorine, chlori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gaseous Diffusion
Gaseous diffusion is a technology that was used to produce enriched uranium by forcing gaseous uranium hexafluoride (UF6) through microporous membranes. This produces a slight separation (enrichment factor 1.0043) between the molecules containing uranium-235 (235U) and uranium-238 (238U). By use of a large cascade of many stages, high separations can be achieved. It was the first process to be developed that was capable of producing enriched uranium in industrially useful quantities, but is nowadays considered obsolete, having been superseded by the more-efficient gas centrifuge process (enrichment factor 1.05 to 1.2). Gaseous diffusion was devised by Francis Simon and Nicholas Kurti at the Clarendon Laboratory in 1940, tasked by the MAUD Committee with finding a method for separating uranium-235 from uranium-238 in order to produce a bomb for the British Tube Alloys project. The prototype gaseous diffusion equipment itself was manufactured by Metropolitan-Vickers (MetroVick ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
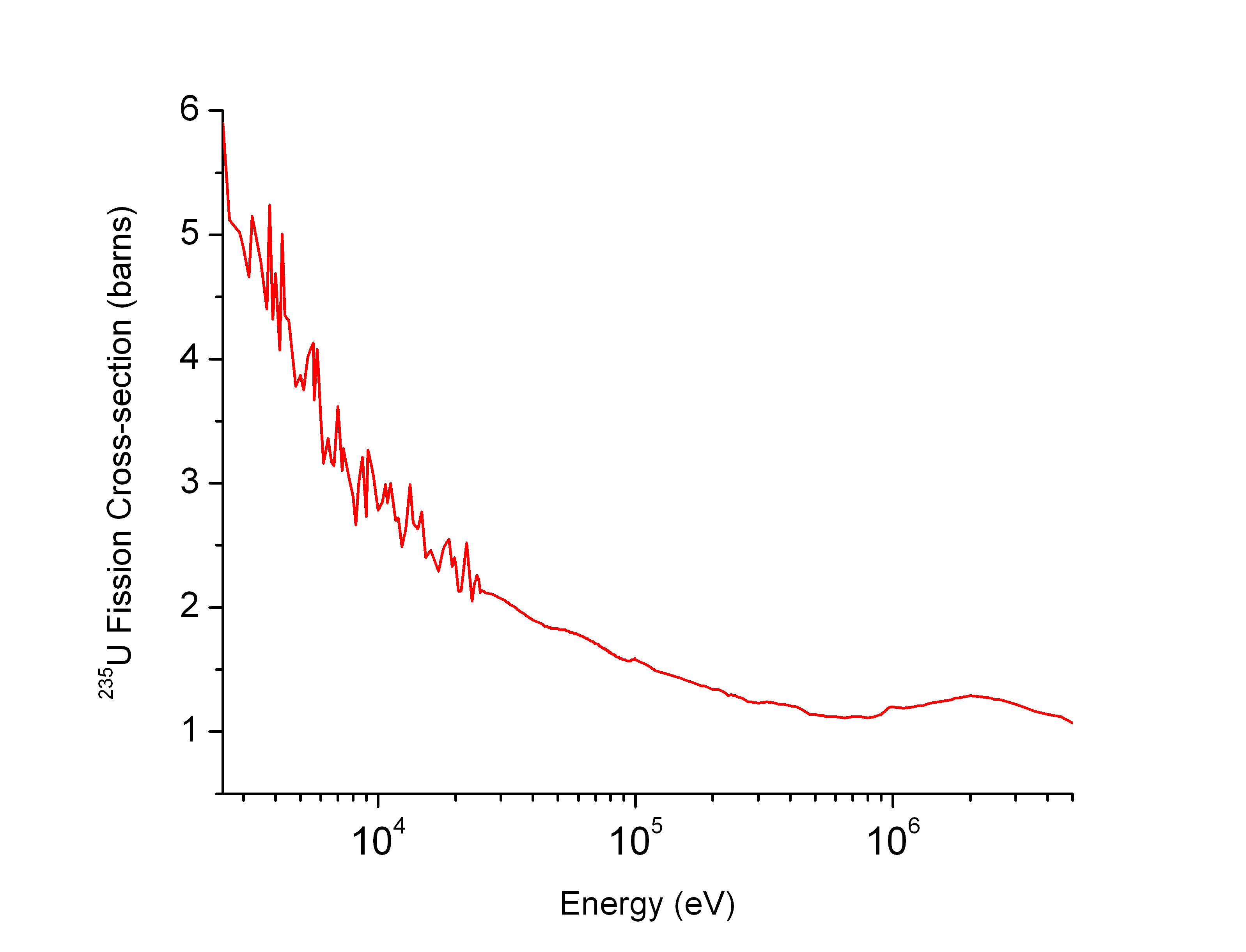
Isotope Separation
Isotope separation is the process of concentrating specific isotopes of a chemical element by removing other isotopes. The use of the nuclides produced is varied. The largest variety is used in research (e.g. in chemistry where atoms of "marker" nuclide are used to figure out reaction mechanisms). By tonnage, separating natural uranium into enriched uranium and depleted uranium is the largest application. In the following text, mainly uranium enrichment is considered. This process is crucial in the manufacture of uranium fuel for nuclear power plants and is also required for the creation of uranium-based nuclear weapons (unless uranium-233 is used). Plutonium-based weapons use plutonium produced in a nuclear reactor, which must be operated in such a way as to produce plutonium already of suitable isotopic mix or ''grade''. While chemical elements can be purified through chemical processes, isotopes of the same element have nearly identical chemical properties which makes this type ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
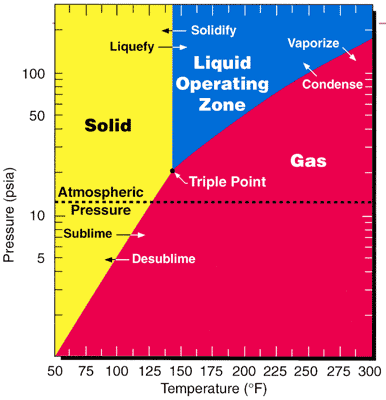
Uranium Hexafluoride
Uranium hexafluoride, sometimes called hex, is the inorganic compound with the formula . Uranium hexafluoride is a volatile, white solid that is used in enriching uranium for nuclear reactors and nuclear weapons. Preparation Uranium dioxide is converted with hydrofluoric acid (HF) to uranium tetrafluoride: : The resulting is subsequently oxidized with fluorine to give the hexafluoride: : In samples contaminated with uranium trioxide, uranyl fluoride, an oxyfluoride compound is produced in the HF step: : which can be fluorinated to produce the same product, uranium hexafluoride. : The fluorination step in both reactions above are highly exothermic. Properties Physical properties At atmospheric pressure, sublimes at 56.5 °C. The solid-state structure was determined by neutron diffraction at 77 K and 293 K.J. C. Taylor, P. W. Wilson, J. W. Kelly: „The structures of fluorides. I. Deviations from ideal symmetry in the structure of crystalline UF6: a neutron diffract ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extremely Reactivity (chemistry), reactive as it reacts with all other Periodic table, elements except for the light Noble gas, noble gases. It is highly toxicity, toxic. Among the elements, fluorine ranks Abundance of the chemical elements, 24th in cosmic abundance and 13th in crustal abundance. Fluorite, the primary mineral source of fluorine, which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Uranium
Natural uranium (NU or Unat) is uranium with the same isotopic ratio as found in nature. It contains 0.711% uranium-235, 99.284% uranium-238, and a trace of uranium-234 by weight (0.0055%). Approximately 2.2% of its radioactivity comes from uranium-235, 48.6% from uranium-238, and 49.2% from uranium-234. Natural uranium can be used to fuel both low- and high-power nuclear reactors. Historically, graphite-moderated reactors and heavy water-moderated reactors have been fueled with natural uranium in the pure metal (U) or uranium dioxide (UO2) ceramic forms. However, experimental fuelings with uranium trioxide (UO3) and triuranium octaoxide (U3O8) have shown promise. The 0.72% uranium-235 is not sufficient to produce a self-sustaining critical chain reaction in light water reactors or nuclear weapons; these applications must use enriched uranium. Nuclear weapons take a concentration of 90% uranium-235, and light water reactors require a concentration of roughly 3% uranium-235 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pressurised Heavy Water Reactor
A pressurized heavy-water reactor (PHWR) is a nuclear reactor that uses heavy water (deuterium oxide D2O) as its coolant and neutron moderator. PHWRs frequently use natural uranium as fuel, but sometimes also use very low enriched uranium. The heavy water coolant is kept under pressure to avoid boiling, allowing it to reach higher temperature (mostly) without forming steam bubbles, exactly as for a pressurized water reactor (PWR). While heavy water is very expensive to isolate from ordinary water (often referred to as ''light water'' in contrast to ''heavy water''), its low absorption of neutrons greatly increases the neutron economy of the reactor, avoiding the need for enriched fuel. The high cost of the heavy water is offset by the lowered cost of using natural uranium and/or alternative fuel cycles. As of the beginning of 2001, 31 PHWRs were in operation, having a total capacity of 16.5 GW(e), representing roughly 7.76% by number and 4.7% by generating capacity of all curren ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Fuel
Nuclear fuel refers to any substance, typically fissile material, which is used by nuclear power stations or other atomic nucleus, nuclear devices to generate energy. Oxide fuel For fission reactors, the fuel (typically based on uranium) is usually based on the metal oxide; the oxides are used rather than the metals themselves because the oxide melting point is much higher than that of the metal and because it cannot burn, being already in the oxidized state. Uranium dioxide Uranium dioxide is a black semiconductor, semiconducting solid. It can be made by heating uranyl nitrate to form . : This is then converted by heating with hydrogen to form UO2. It can be made from Enriched uranium, enriched uranium hexafluoride by reacting with ammonia to form a solid called ammonium diuranate, . This is then heated (Calcination, calcined) to form and U3O8 which is then converted by heating with hydrogen or ammonia to form UO2. The UO2 is mixed with an organic binder and pressed in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Reactor
A nuclear reactor is a device used to initiate and control a Nuclear fission, fission nuclear chain reaction. They are used for Nuclear power, commercial electricity, nuclear marine propulsion, marine propulsion, Weapons-grade plutonium, weapons production and Research reactor, research. Fissile material, Fissile nuclei (primarily uranium-235 or plutonium-239) absorb single neutron, neutrons and split, releasing energy and multiple neutrons, which can induce further fission. Reactors stabilize this, regulating Neutron absorber, neutron absorbers and neutron moderator, moderators in the core. Fuel efficiency is exceptionally high; Enriched uranium#Low-enriched uranium (LEU), low-enriched uranium is 120,000 times more energy dense than coal. Heat from nuclear fission is passed to a working fluid Nuclear reactor#By coolant, coolant. In commercial reactors, this drives Turbine, turbines and electrical generator shafts. Some reactors are used for district heating, and isotopes, isoto ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
JOM (journal)
''JOM'' is a technical journal devoted to exploring the many aspects of materials, science and engineering published monthly by The Minerals, Metals & Materials Society (TMS) (a member-based professional society). ''JOM'' reports scholarly work that explores the many aspects of materials science and engineering within the broad topical areas of light metals, structural materials, functional materials, extraction and processing, and materials processing and manufacturing. ''JOM'' strives to balance the interests of the laboratory and the marketplace by reporting academic, industrial, and government-sponsored work from around the world. History From 1949 through 1988, the journal was named ''Journal of Metals''. With materials systems becoming commonplace and with the journal frequently covering composites, plastics, and other materials, its name was changed to ''JOM''. It is published by TMS, which is headquartered in Pittsburgh, Pennsylvania Pittsburgh ( ) is a cit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |