|
Xylindein
Xylindein is a quinone pigment, a dimeric naphthoquinone derivative. It is produced by fungi in the genus ''Chlorociboria''. This pigment causes green staining of wood infected by the fungi. Etymology This pigment was firstly extracted in 1868 by Paul Thénard from wood and resembled indigo, so he called it . Combination of ''wikt:xylo-, xyl-'' (wood) and ''wikt:Inde, indé'' (indigo) + ''wikt:-ine, -ine''. References * * * * External links * {{Commonscat-inline, Xylindein Fungal pigments Heterocyclic compounds with 7 or more rings Natural phenol dimers Dihydroisocoumarins Pyrans Hydroxyarenes 3-Hydroxypropenals within hydroxyquinones Polyenes Naphthoquinones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chlorociboria
''Chlorociboria'' is the type genus of in the fungal family (biology), family Chlorociboriaceae within order Helotiales. The genus includes 23 species. Two common temperate zone species, ''Chlorociboria aeruginascens'' and ''Chlorociboria aeruginosa'', can only reliably be distinguished by microscopic examination. ''Chlorociboria aeruginosa'' has larger ascospore, spores (9–15 micrometre, μm × 1.5–2.5 μm) and the worm-like cells of the outer surface are rough, unlike the commoner ''C. aeruginascens'', of which the spores are 6–10 μm × 1.5–2 μm. The hyphae and ascocarp, fruit bodies of all species make xylindein, a secondary metabolite that stains the Substrate (biology), substrate wood blue-green, with "green oak" being a valued commodity in woodworking. The blue-green pigmented wood is featured in Tunbridge ware. Habit Blue-green stain is evident year-round, with ascocarp production occurring from summer to fall. Species *''Chlorociboria ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully Conjugated system, conjugated cyclic diketone, dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone" (thus the name of the class). Other important examples are 1,2-benzoquinone (''ortho''-quinone), 1,4-naphthoquinone and 9,10-anthraquinone. The name is derived from that of quinic acid (with the suffix "-one" indicating a ketone), since it is one of the compounds obtained upon oxidation of quinic acid. Quinic acid, like quinine is obtained from cinchona bark, called quinaquina in the indigenous languages of Peruvian tribes. Properties Quinones are oxidized derivatives of aromatic compounds an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
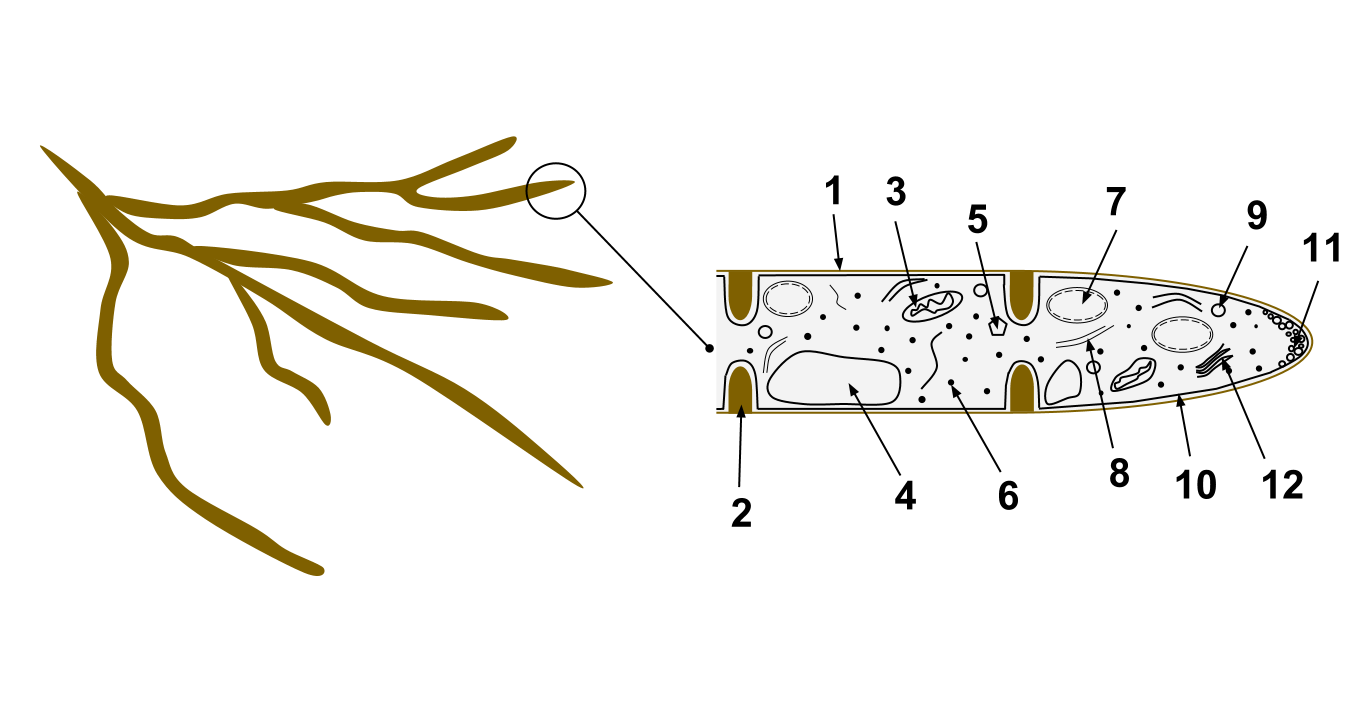
Fungal Pigments
A fungus (: fungi , , , or ; or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and molds, as well as the more familiar mushrooms. These organisms are classified as one of the traditional eukaryotic kingdoms, along with Animalia, Plantae, and either Protista or Protozoa and Chromista. A characteristic that places fungi in a different kingdom from plants, bacteria, and some protists is chitin in their cell walls. Fungi, like animals, are heterotrophs; they acquire their food by absorbing dissolved molecules, typically by secreting digestive enzymes into their environment. Fungi do not photosynthesize. Growth is their means of mobility, except for spores (a few of which are flagellated), which may travel through the air or water. Fungi are the principal decomposers in ecological systems. These and other differences place fungi in a single group of related organisms, named the ''Eumycota'' (''true fungi'' or ''Eumycetes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxyarenes
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. It is acutely toxic and is considered a health hazard. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 million tonnes a year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds, and is a liquid when manufactured. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, explosives such as picric acid, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical dru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrans
In chemistry, pyran is a six-membered heterocyclic, non-aromatic ring, consisting of five carbon atoms and one oxygen atom and containing two double bonds. The molecular formula is C5H6O. There are two isomers of pyran that differ by the location of the double bonds. In 2''H''-pyran, the saturated carbon is at position 2, whereas, in 4''H''-pyran, the saturated carbon is at position 4. "Oxine” is not used for pyran because it has been used as a trivial name for quinolin-8-ol. 4''H''-Pyran was first isolated and characterized in 1962 via pyrolysis of 2-acetoxy-3,4-dihydro-2''H''-pyran. It was found to be unstable, particularly in the presence of air. 4''H''-pyran easily disproportionates to the corresponding dihydropyran and the pyrylium ion, which is easily hydrolyzed in aqueous medium. Although the pyrans themselves have little significance in chemistry, many of their derivatives are important biological molecules, such as the pyranoflavonoids. The term pyran is also often app ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydroisocoumarins
Dihydroisocoumarins are phenolic compounds related to isocoumarin. Dihydroisocoumarin glucosides A glucoside is a glycoside that is chemically derived from glucose. Glucosides are common in plants, but rare in animals. Glucose is produced when a glucoside is Hydrolysis, hydrolysed by purely chemical means, or decomposed by fermentation (bio ... can be found in '' Caryocar glabrum''. References {{heterocyclic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Natural Phenol Dimers
Nature is an inherent character or constitution, particularly of the ecosphere or the universe as a whole. In this general sense nature refers to the laws, elements and phenomena of the physical world, including life. Although humans are part of nature, human activity or humans as a whole are often described as at times at odds, or outright separate and even superior to nature. During the advent of modern scientific method in the last several centuries, nature became the passive reality, organized and moved by divine laws. With the Industrial Revolution, nature increasingly became seen as the part of reality deprived from intentional intervention: it was hence considered as sacred by some traditions ( Rousseau, American transcendentalism) or a mere decorum for divine providence or human history (Hegel, Marx). However, a vitalist vision of nature, closer to the pre-Socratic one, got reborn at the same time, especially after Charles Darwin. Within the various uses of the word ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heterocyclic Compounds With 7 Or More Rings
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, properties, and applications of organic heterocycles. Examples of heterocyclic compounds include all of the nucleic acids, the majority of drugs, most biomass (cellulose and related materials), and many natural and synthetic dyes. More than half of known compounds are heterocycles. 59% of US FDA-approved drugs contain nitrogen heterocycles. Classification The study of organic heterocyclic chemistry focuses especially on organic unsaturated derivatives, and the preponderance of work and applications involves unstrained organic 5- and 6-membered rings. Included are pyridine, thiophene, pyrrole, and furan. Another large class of organic heterocycles refers to those fused to benzene rings. For example, the fused benzene derivatives of pyridin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Comptes Rendus De L'Académie Des Sciences
(, ''Proceedings of the Academy of Sciences''), or simply ''Comptes rendus'', is a French scientific journal published since 1835. It is the proceedings of the French Academy of Sciences. It is currently split into seven sections, published on behalf of the Academy until 2020 by Elsevier: ''Mathématique, Mécanique, Physique, Géoscience, Palévol, Chimie, ''and'' Biologies.'' As of 2020, the ''Comptes Rendus'' journals are published by the Academy with a diamond open access model. Naming history The journal has had several name changes and splits over the years. 1835–1965 ''Comptes rendus'' was initially established in 1835 as ''Comptes rendus hebdomadaires des séances de l'Académie des Sciences''. It began as an alternative publication pathway for more prompt publication than the ''Mémoires de l'Académie des Sciences,'' which had been published since 1666. The ''Mémoires,'' which continued to be published alongside the ''Comptes rendus'' throughout the ninetee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pigment
A pigment is a powder used to add or alter color or change visual appearance. Pigments are completely or nearly solubility, insoluble and reactivity (chemistry), chemically unreactive in water or another medium; in contrast, dyes are colored substances which are soluble or go into solution at some stage in their use. Dyes are often organic compounds whereas pigments are often inorganic compound, inorganic. Pigments of prehistoric and historic value include ochre, charcoal, and lapis lazuli. Economic impact In 2006, around 7.4 million tons of inorganic chemistry, inorganic, organic chemistry, organic, and special pigments were marketed worldwide. According to an April 2018 report by ''Bloomberg Businessweek'', the estimated value of the pigment industry globally is $30 billion. The value of titanium dioxide – used to enhance the white brightness of many products – was placed at $13.2 billion per year, while the color Ferrari red is valued at $300 million each yea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
-ine
''-ine'' is a suffix used in chemistry to denote two kinds of substance. The first is a chemically basic and alkaloidal substance. It was proposed by Joseph Louis Gay-Lussac in an editorial accompanying a paper by Friedrich Sertürner describing the isolation of the alkaloid "morphium", which was subsequently renamed to "morphine".Sneader W. (2005). ''Drug Discovery: A History'', pp. 90-91. Wiley. Examples include quinine, morphine and guanidine. The second usage is to denote a hydrocarbon of the second degree of unsaturation. Examples include hexine and heptine. With simple hydrocarbons, this usage is identical to the IUPAC suffix -yne. In common and literary adjectives (e.g. ''asinine, canine, feline, ursine''), the suffix is usually pronounced or in some words alternatively . For demonyms (e.g. ''Levantine, Byzantine, Argentine'') it is usually or . But in chemistry, it is usually pronounced or depending on the word it appears in and the accent of the speaker. In a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |