|
Topoisomerase Inhibitor
Topoisomerase inhibitors are chemical compounds that block the action of topoisomerases, which are broken into two broad subtypes: type I topoisomerases (TopI) and type II topoisomerases (TopII). Topoisomerase plays important roles in cellular reproduction and DNA organization, as they mediate the cleavage of single and double stranded DNA to relax supercoils, untangle catenanes, and condense chromosomes in eukaryotic cells. Topoisomerase inhibitors influence these essential cellular processes. Some topoisomerase inhibitors prevent topoisomerases from performing DNA strand breaks while others, deemed topoisomerase poisons, associate with topoisomerase-DNA complexes and prevent the re-ligation step of the topoisomerase mechanism. These topoisomerase-DNA-inhibitor complexes are cytotoxic agents, as the un-repaired single- and double stranded DNA breaks they cause can lead to apoptosis and cell death. Because of this ability to induce apoptosis, topoisomerase inhibitors have gained i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Topoisomerase
DNA topoisomerases (or topoisomerases) are enzymes that catalyze changes in the topological state of DNA, interconverting relaxed and supercoiled forms, linked (catenated) and unlinked species, and knotted and unknotted DNA. Topological issues in DNA arise due to the intertwined nature of its double-helical structure, which, for example, can lead to overwinding of the DNA duplex during DNA replication and transcription. If left unchanged, this torsion would eventually stop the DNA or RNA polymerases involved in these processes from continuing along the DNA helix. A second topological challenge results from the linking or tangling of DNA during replication. Left unresolved, links between replicated DNA will impede cell division. The DNA topoisomerases prevent and correct these types of topological problems. They do this by binding to DNA and cutting the sugar-phosphate backbone of either one (type I topoisomerases) or both (type II topoisomerases) of the DNA strands. This transien ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Indolocarbazole
Indolocarbazoles (ICZs) are a class of compounds that are under current study due to their potential as anti-cancer as well as antimicrobial drugs and the prospective number of derivatives and uses found from the basic backbone alone. First isolated in 1977, a wide range of structures and derivatives have been found or developed throughout the world. Due to the extensive number of structures available, this review will focus on the more important groups here while covering their occurrence, biological activity, biosynthesis, and laboratory synthesis. Chemical classification Indolocarbazoles belong to the alkaloid sub-class of bisindoles. The most frequently isolated indolocarbazoles are Indolo(2,3-a)carbazoles; the most common subgroup of the Indolo(2,3-a)carbazoles are the Indolo(2,3-a)pyrrole(3,4-c)carbazoles. These can be divided into two major classes - halogenated (chlorinated) with a fully oxidized C-7 carbon with only one indole nitrogen containing a β-glycosidic bond an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
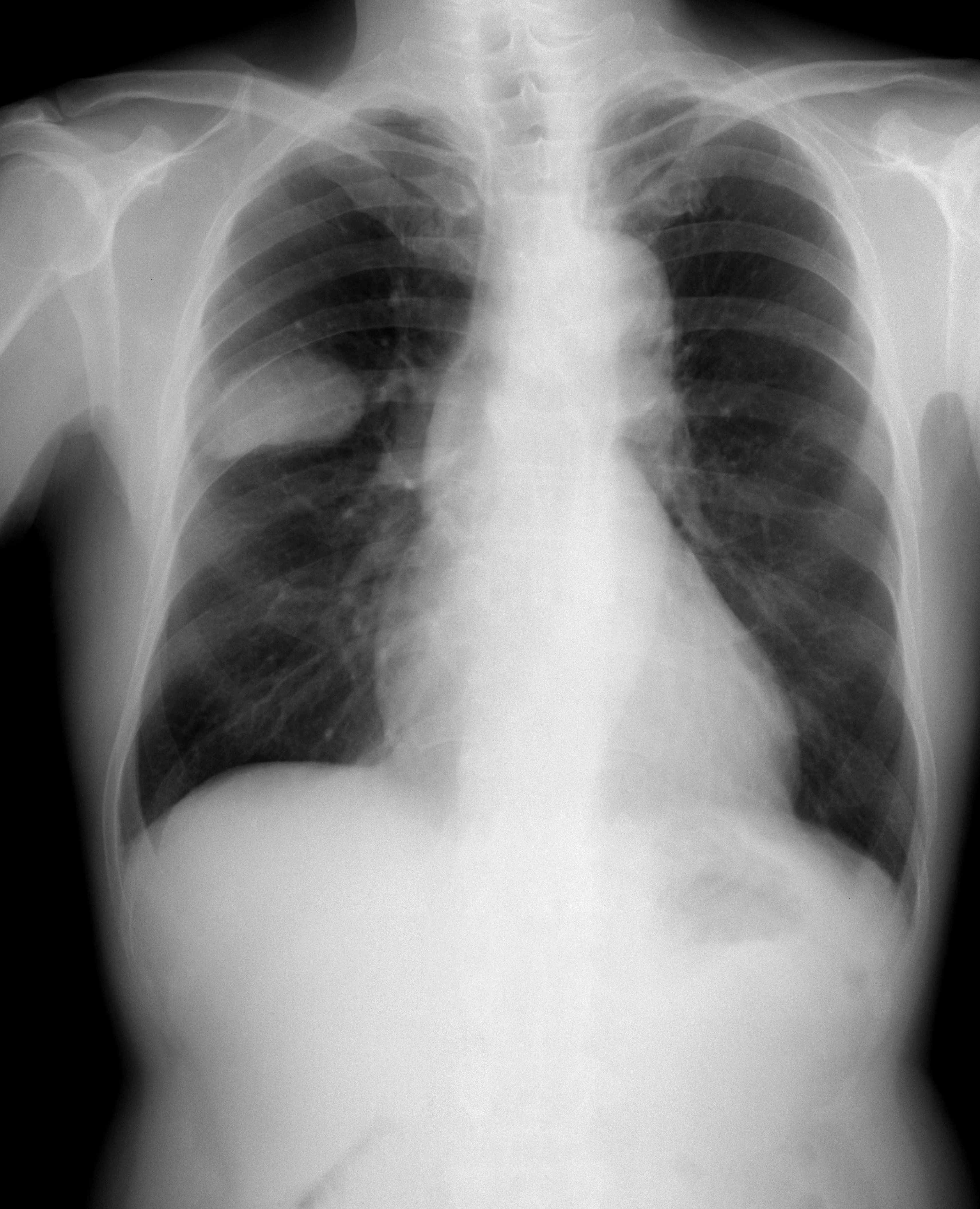
Small Cell Lung Cancer
Small-cell carcinoma, also known as oat cell carcinoma, is a type of highly malignant cancer that most commonly arises within the lung, although it can occasionally arise in other body sites, such as the cervix, prostate, and gastrointestinal tract. Compared to non-small cell carcinoma, small cell carcinoma is more aggressive, with a shorter doubling time, higher growth fraction, and earlier development of metastases. Extensive stage small cell lung cancer (SCLC) is classified as a rare disorder. Ten-year relative survival rate (combined limited and extensive SCLC) is 3.5% (4.3% for women, 2.8% for men). Survival can be higher or lower based on a combination of factors including stage, age, sex and race. While all lung cancers are associated with tobacco smoking, SCLC is very strongly associated with tobacco smoking. Types Lung cancer Small-cell lung carcinoma (SCLC) has long been divided into two clinicopathological stages, termed limited stage (LS) and extensive stage (ES). ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Belotecan
Belotecan is a drug used in chemotherapy. It is a semi-synthetic camptothecin analogue indicated for small-cell lung cancer and ovarian cancer Ovarian cancer is a cancerous tumor of an ovary. It may originate from the ovary itself or more commonly from communicating nearby structures such as fallopian tubes or the inner lining of the abdomen. The ovary is made up of three different ..., approved in South Korea under the trade name Camtobell, presented in 2 mg vials for injection. The drug has been marketed by Chong Kun Dang Pharmaceuticals since 2003. Mechanism of action Belotecan blocks topoisomerase I with a pIC50 of 6.56, stabilizing the cleavable complex of topoisomerase I-DNA, which inhibits the religation of single-stranded DNA breaks generated by topoisomerase I; lethal double-stranded DNA breaks occur when the topoisomerase I-DNA complex is encountered by the DNA replication machinery, DNA replication is disrupted, and the tumor cell undergoes apoptosis. T ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Topotecan
Topotecan, sold under the brand name Hycamtin among others, is a chemotherapeutic agent medication that is a topoisomerase inhibitor. It is a synthetic, water-soluble analog of the natural chemical compound camptothecin. It is used in the form of its hydrochloride salt to treat ovarian cancer, lung cancer and other cancer types. After GlaxoSmithKline received final FDA approval for topotecan on 15 October 2007, it became the first topoisomerase I inhibitor for oral use. Uses * Ovarian cancer (FDA May 1996). * Cervical cancer (FDA June 2006). * Small cell lung carcinoma (SCLC) (FDA Oct 2007). Experimental uses As of 2016, experiments were under way for neuroblastoma, brainstem glioma, Ewing's sarcoma and Angelman's syndrome. In addition, topotecan is experimentally treating non-small cell lung cancer, colorectal cancer-, breast cancer, non-Hodgkin lymphoma, endometrial cancer, and oligodendroglioma. Angelman's syndrome Angelman's syndrome is a neuro-genetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring the angles and intensities of the X-ray diffraction, a crystallography, crystallographer can produce a three-dimensional picture of the density of electrons within the crystal and the positions of the atoms, as well as their chemical bonds, crystallographic disorder, and other information. X-ray crystallography has been fundamental in the development of many scientific fields. In its first decades of use, this method determined the size of atoms, the lengths and types of chemical bonds, and the atomic-scale differences between various materials, especially minerals and alloys. The method has also revealed the structure and function of many biological molecules, including vitamins, drugs, proteins and nucleic acids such as DNA. X-ray crystall ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DNA Intercalation
In biochemistry, intercalation is the insertion of molecules between the planar bases of deoxyribonucleic acid (DNA). This process is used as a method for analyzing DNA and it is also the basis of certain kinds of poisoning. There are several ways molecules (in this case, also known as ''ligand (biochemistry), ligands'') can interact with DNA. Ligands may interact with DNA by Covalent bond, covalently binding, electrostatically binding, or intercalating. Intercalation occurs when ligands of an appropriate size and chemical nature fit themselves in between base pairs of DNA. These ligands are mostly polycyclic, aromatic, and planar, and therefore often make good nucleic acid staining (biology), stains. Intensively studied DNA intercalators include berberine, ethidium bromide, proflavine, daunomycin, doxorubicin, and thalidomide. DNA intercalators are used in chemotherapy, chemotherapeutic treatment to inhibit DNA replication in rapidly growing cancer cells. Examples include doxo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water is the nucleophile. Biological hydrolysis is the cleavage of Biomolecule, biomolecules where a water molecule is consumed to effect the separation of a larger molecule into component parts. When a carbohydrate is broken into its component sugar molecules by hydrolysis (e.g., sucrose being broken down into glucose and fructose), this is recognized as saccharification. Hydrolysis reactions can be the reverse of a condensation reaction in which two molecules join into a larger one and eject a water molecule. Thus hydrolysis adds water to break down, whereas condensation builds up by removing water. Types Usually hydrolysis is a chemical process in which a molecule of water is added to a substance. Sometimes this addition causes both the su ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactone
Lactones are cyclic carboxylic esters. They are derived from the corresponding hydroxycarboxylic acids by esterification. They can be saturated or unsaturated. Lactones are formed by lactonization, the intramolecular esterification of the corresponding hydroxycarboxylic acids. Nomenclature Greek alphabet#Letters, Greek prefixes in alphabetical order indicate ring size. Lactones are usually named according to the precursor acid molecule (''aceto'' = 2 carbon atoms, ''propio'' = 3, ''butyro'' = 4, ''valero'' = 5, ''capro'' = 6, etc.), with a ''-lactone'' suffix and a Greek letter prefix that specifies the number of carbon atoms in the heterocycle — that is, the distance between the relevant -OH and the -COOH groups along said backbone. The first carbon atom after the carbon in the -COOH group on the parent compound is labelled α, the second will be labeled β, and so forth. Therefore, the prefixes also indicate the size of the lactone ring: α-lactone = 3-membered ring, β-lac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Camptothecin
Camptothecin (CPT) is a topoisomerase inhibitor. It was discovered in 1966 by M. E. Wall and M. C. Wani in systematic screening of natural products for anticancer drugs. It was isolated from the Bark (botany), bark of ''Camptotheca acuminata'' (喜树 "Happy tree"), a tree native to China used in traditional Chinese medicine. It has been used clinically in China for the treatment of gastrointestinal tumors. CPT showed anticancer activity in preliminary clinical trials, especially against breast, ovarian, colon, lung, and stomach cancers. However, it has low solubility and adverse effects have been reported when used therapeutically, so synthetic and medicinal chemists have developed numerous syntheses of camptothecin and various derivatives to increase the benefits of the chemical, with good results. Four CPT Analog (chemistry), analogues have been approved and are used in cancer chemotherapy today: topotecan, irinotecan, belotecan, and trastuzumab deruxtecan. Camptothecin has als ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligation (molecular Biology)
Ligation is the joining of two nucleotides, or two nucleic acid fragments, into a single polymeric chain through the action of an enzyme known as a ligase. The reaction involves the formation of a phosphodiester bond between the 3'-hydroxyl terminus of one nucleotide and the 5'-phosphoryl terminus of another nucleotide, which results in the two nucleotides being linked consecutively on a single strand. Ligation works in fundamentally the same way for both DNA and RNA. A cofactor is generally involved in the reaction, usually Adenosine triphosphate, ATP or Nicotinamide riboside, NAD+. Eukaryotic ligases belong to the ATP type, while the NAD+ type are found in bacteria (e.g. Escherichia coli, ''E. coli''). Ligation occurs naturally as part of numerous cellular processes, including DNA replication, transcription, splicing, and recombination, and is also an essential laboratory procedure in molecular cloning, whereby DNA fragments are joined to create recombinant DNA molecules (such a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
DNA Supercoil
DNA supercoiling refers to the amount of twist in a particular DNA strand, which determines the amount of strain on it. A given strand may be "positively supercoiled" or "negatively supercoiled" (more or less tightly wound). The amount of a strand's supercoiling affects a number of biological processes, such as compacting DNA and regulating access to the genetic code (which strongly affects DNA metabolism and possibly gene expression). Certain enzymes, such as topoisomerases, change the amount of DNA supercoiling to facilitate functions such as DNA replication and transcription. The amount of supercoiling in a given strand is described by a mathematical formula that compares it to a reference state known as "relaxed B-form" DNA. Overview In a "relaxed" double-helical segment of B-DNA, the two strands twist around the helical axis once every 10.4–10.5 base pairs of sequence. Adding or subtracting twists, as some enzymes do, imposes strain. If a DNA segment under twist s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |