|
Thulium-170
Thulium-170 (170Tm or Tm-170) is a radioactive Isotopes of thulium, isotope of thulium proposed for use in radiotherapy and in radioisotope thermoelectric generators. Properties Thulium-170 has a nuclear binding energy, binding energy of per nucleon and a half-life of . It decays by beta decay, β− decay to Ytterbium-170, 170Yb about 99.869% of the time, and by electron capture to erbium-170, 170Er about 0.131% of the time. About 18.1% of β− decays populate a narrow excited state of 170Yb at (), and this is the main X-ray emission from 170Tm; lower bands are also produced through X-ray fluorescence at 7.42, 51.354, 52.389, 59.159, 59.383, and 60.962 keV. The ground state of thulium-170 has a spin (physics), spin of 1+. The charge radius is , the magnetic moment is , and the electric quadrupole moment is . Proposed applications As a rare-earth element, thulium-170 can be used as the pure metal or thulium hydride, but most commonly thulium oxide due to the refractory ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thulium
Thulium is a chemical element with the symbol Tm and atomic number 69. It is the thirteenth and third-last element in the lanthanide series. Like the other lanthanides, the most common oxidation state is +3, seen in its oxide, halides and other compounds; however, the +2 oxidation state can also be stable. In aqueous solution, like compounds of other late lanthanides, soluble thulium compounds form coordination complexes with nine water molecules. In 1879, the Swedish chemist Per Teodor Cleve separated from the rare earth oxide erbia another two previously unknown components, which he called holmia and thulia; these were the oxides of holmium and thulium, respectively. A relatively pure sample of thulium metal was first obtained in 1911. Thulium is the second-least abundant of the lanthanides, after radioactively unstable promethium which is only found in trace quantities on Earth. It is an easily workable metal with a bright silvery-gray luster. It is fairly soft and slowly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thulium Hydride
Thulium is a chemical element with the symbol Tm and atomic number 69. It is the thirteenth and third-last element in the lanthanide series. Like the other lanthanides, the most common oxidation state is +3, seen in its oxide, halides and other compounds; however, the +2 oxidation state can also be stable. In aqueous solution, like compounds of other late lanthanides, soluble thulium compounds form coordination complexes with nine water molecules. In 1879, the Swedish chemist Per Teodor Cleve separated from the rare earth oxide erbia another two previously unknown components, which he called holmia and thulia; these were the oxides of holmium and thulium, respectively. A relatively pure sample of thulium metal was first obtained in 1911. Thulium is the second-least abundant of the lanthanides, after radioactively unstable promethium which is only found in trace quantities on Earth. It is an easily workable metal with a bright silvery-gray luster. It is fairly soft and s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Radioisotope
A synthetic radioisotope is a radionuclide that is not found in nature: no natural process or mechanism exists which produces it, or it is so unstable that it decays away in a very short period of time. Examples include technetium-95 and promethium-146. Many of these are found in, and harvested from, spent nuclear fuel assemblies. Some must be manufactured in particle accelerators. Production Some synthetic radioisotopes are extracted from spent nuclear reactor fuel rods, which contain various fission products. For example, it is estimated that up to 1994, about 49,000 terabecquerels (78 metric ton) of technetium was produced in nuclear reactors, which is by far the dominant source of terrestrial technetium. Some synthetic isotopes are produced in significant quantities by fission but are not yet being reclaimed. Other isotopes are manufactured by neutron irradiation of parent isotopes in a nuclear reactor (for example, Tc-97 can be made by neutron irradiation of Ru-96) or by b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nuclear Magneton
The nuclear magneton (symbol ''μ'') is a physical constant of magnetic moment, defined in SI units by: :\mu_\text = and in Gaussian CGS units by: :\mu_\text = where: :''e'' is the elementary charge, :''ħ'' is the reduced Planck constant, :''m'' is the proton rest mass, and :''c'' is the speed of light In SI units, its value is approximately: :''μ'' = In Gaussian CGS units, its value can be given in convenient units as :''μ'' = The nuclear magneton is the natural unit for expressing magnetic dipole moments of heavy particles such as nucleons and atomic nuclei. Due to the fact that neutrons and protons consist of quarks and thus are not really Dirac particles, their magnetic moments differ from ''μ'': :\mu_\text = 2793 \mu_\text :\mu_\text = -1913 \mu_\text The magnetic dipole moment of the electron, which is much larger as a consequence of much larger charge-to-mass ratio, is usually expressed in units of the ''Bohr magneton'', which is calculated in the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bremsstrahlung
''Bremsstrahlung'' (), from "to brake" and "radiation"; i.e., "braking radiation" or "deceleration radiation", is electromagnetic radiation produced by the deceleration of a charged particle when deflected by another charged particle, typically an electron by an atomic nucleus. The moving particle loses kinetic energy, which is converted into radiation (i.e., photons), thus satisfying the law of conservation of energy. The term is also used to refer to the process of producing the radiation. ''Bremsstrahlung'' has a continuous spectrum, which becomes more intense and whose peak intensity shifts toward higher frequencies as the change of the energy of the decelerated particles increases. Broadly speaking, ''bremsstrahlung'' or braking radiation is any radiation produced due to the deceleration (negative acceleration) of a charged particle, which includes synchrotron radiation (i.e., photon emission by a relativistic particle), cyclotron radiation (i.e. photon emission by a n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Steelmaking
Steelmaking is the process of producing steel from iron ore and carbon/or scrap. In steelmaking, impurities such as nitrogen, silicon, phosphorus, sulfur and excess carbon (the most important impurity) are removed from the sourced iron, and alloying elements such as manganese, nickel, chromium, carbon and vanadium are added to produce different grades of steel. Limiting dissolved gases such as nitrogen and oxygen and entrained impurities (termed "inclusions") in the steel is also important to ensure the quality of the products cast from the liquid steel. Steelmaking has existed for millennia, but it was not commercialized on a massive scale until the mid- 19th century. An ancient process of steelmaking was the crucible process. In the 1850s and 1860s, the Bessemer process and the Siemens-Martin process turned steelmaking into a heavy industry. Today there are two major commercial processes for making steel, namely basic oxygen steelmaking, which has liquid pig-iron from the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiography
Radiography is an imaging technique using X-rays, gamma rays, or similar ionizing radiation and non-ionizing radiation to view the internal form of an object. Applications of radiography include medical radiography ("diagnostic" and "therapeutic") and industrial radiography. Similar techniques are used in airport security (where "body scanners" generally use backscatter X-ray). To create an image in conventional radiography, a beam of X-rays is produced by an X-ray generator and is projected toward the object. A certain amount of the X-rays or other radiation is absorbed by the object, dependent on the object's density and structural composition. The X-rays that pass through the object are captured behind the object by a detector (either photographic film or a digital detector). The generation of flat two dimensional images by this technique is called projectional radiography. In computed tomography (CT scanning) an X-ray source and its associated detectors rotate around ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Energy Research Establishment
The Atomic Energy Research Establishment (AERE) was the main Headquarters, centre for nuclear power, atomic energy research and development in the United Kingdom from 1946 to the 1990s. It was created, owned and funded by the British Government. A number of early research reactors were built here starting with GLEEP in 1947 to provide the underlying science and technology behind the design and building of Britain's nuclear reactors such as the Windscale Piles and Calder Hall nuclear power station. To support this an extensive array of research and design laboratories were built to enable research into all aspects of nuclear reactor and fuel design, and the development of pilot plants for fuel reprocessing. The site became a major employer in the Oxford area. In the 1990s demand for government-led research had significantly decreased and the site was subsequently gradually diversified to allow private investment, and was known from 2006 as the Harwell Science and Innovati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
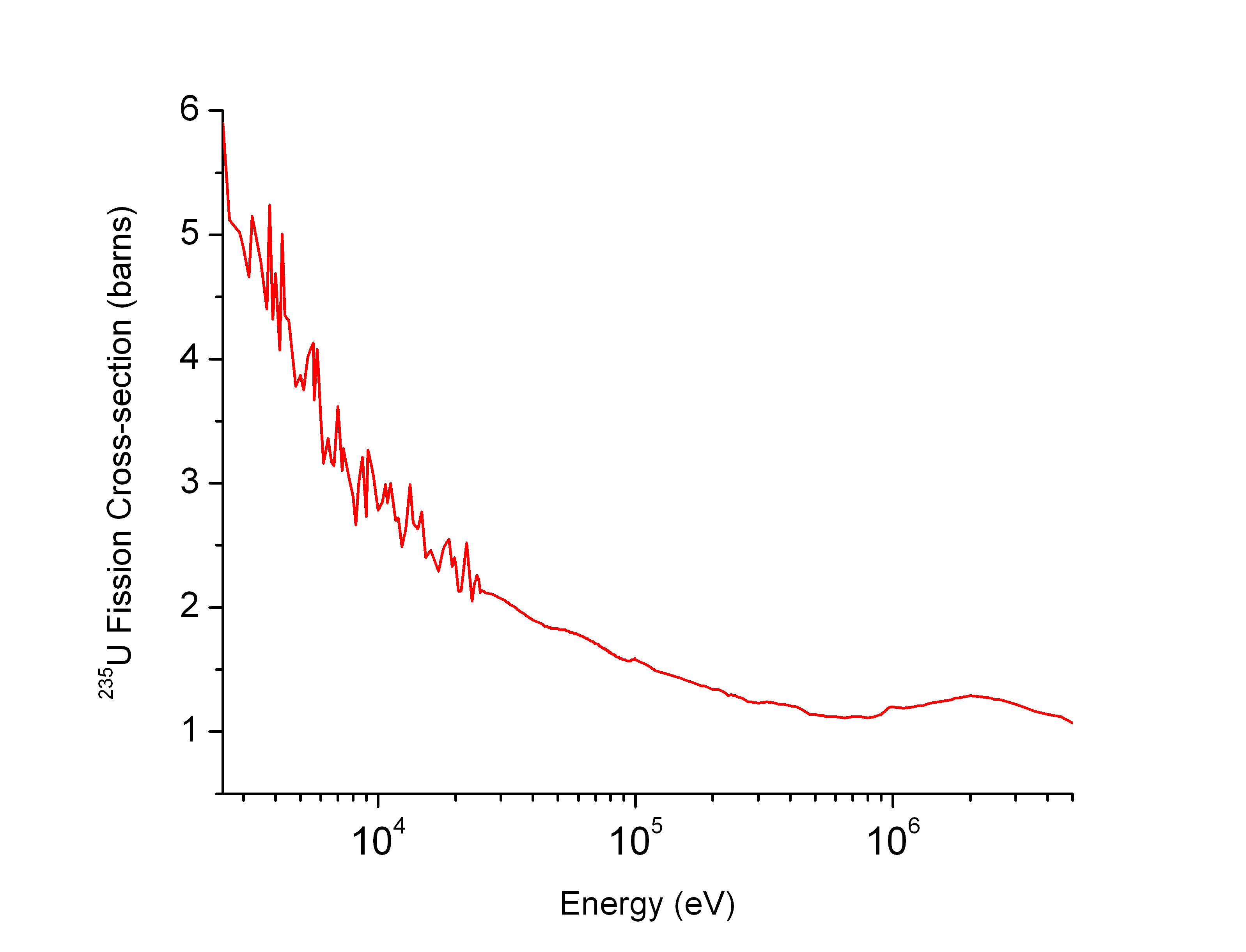
Neutron Capture Cross Section
In nuclear physics, the concept of a neutron cross section is used to express the likelihood of interaction between an incident neutron and a target nucleus. The neutron cross section σ can be defined as the area in cm2 for which the number of neutron-nuclei reactions taking place is equal to the product of the number of incident neutrons that would pass through the area and the number of target nuclei. In conjunction with the neutron flux, it enables the calculation of the reaction rate, for example to derive the thermal power of a nuclear power plant. The standard unit for measuring the cross section is the barn, which is equal to 10−28 m2 or 10−24 cm2. The larger the neutron cross section, the more likely a neutron will react with the nucleus. An isotope (or nuclide) can be classified according to its neutron cross section and how it reacts to an incident neutron. Nuclides that tend to absorb a neutron and either decay or keep the neutron in its nucleus are neutron ab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Neutron Irradiation
Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus decays immediately by emitting gamma rays, or particles such as beta particles, alpha particles, fission products, and neutrons (in nuclear fission). Thus, the process of neutron capture, even after any intermediate decay, often results in the formation of an unstable activation product. Such radioactive nuclei can exhibit half-lives ranging from small fractions of a second to many years. Neutron activation is the only common way that a stable material can be induced into becoming intrinsically radioactive. All naturally occurring materials, including air, water, and soil, can be induced (activated) by neutron capture into some amount of radioactivity in varying degrees, as a result of the production of neutron-rich radioisotopes. Some atoms require more than one n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Refractory
In materials science, a refractory material or refractory is a material that is resistant to decomposition by heat, pressure, or chemical attack, and retains strength and form at high temperatures. Refractories are polycrystalline, polyphase, inorganic, non-metallic, porous, and heterogeneous. They are typically composed of oxides or carbides, nitrides etc. of the following materials: silicon, aluminium, magnesium, calcium, boron, chromium and zirconium. ASTM C71 defines refractories as "...non-metallic materials having those chemical and physical properties that make them applicable for structures, or as components of systems, that are exposed to environments above ." Refractory materials are used in furnaces, kilns, incinerators, and reactors. Refractories are also used to make crucibles and moulds for casting glass and metals and for surfacing flame deflector systems for rocket launch structures. Today, the iron- and steel-industry and metal casting sectors use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thulium Oxide
Thulium(III) oxide is a pale green solid compound, with the formula Tm2 O3. It was first isolated in 1879, from an impure sample of erbia, by Swedish chemist Per Teodor Cleve, who named it ''thulia''. It can be prepared in the laboratory by burning thulium Thulium is a chemical element with the symbol Tm and atomic number 69. It is the thirteenth and third-last element in the lanthanide series. Like the other lanthanides, the most common oxidation state is +3, seen in its oxide, halides and other c ... metal in air, or by decomposition of their oxoacid salts, such as thulium nitrate. References {{Oxides Thulium compounds Sesquioxides ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |



.jpg)