|
Thorocene
Actinocenes are a family of organoactinide compounds consisting of metallocenes containing elements from the actinide series. They typically have a sandwich structure with two dianionic cyclooctatetraenyl ligands (COT2−, which is ) bound to an actinide-metal center (An) in the oxidation state IV, resulting in the general formula An(C8H8)2. Characterised actinocenes The most studied actinocene is uranocene, U(C8H8)2, which in 1968 was the first member of this family to be synthesised and is still viewed as the archetypal example. Other actinocenes that have been synthesised are protactinocene (Pa(C8H8)2), thorocene (Th(C8H8)2), neptunocene (Np(C8H8)2), and plutonocene (Pu(C8H8)2). Especially the latter two, neptunocene and plutonocene, have not been extensively studied experimentally since the 1980s because of the radiation hazard they pose. Berkelocene (with a modified COT ligand) was synthesised in 2025, the first actinocene with a new actinide in over 50 years. Bonding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
|
Thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and has a high melting point. Thorium is an electropositive actinide whose chemistry is dominated by the +4 oxidation state; it is quite reactive and can ignite in air when finely divided. All known thorium isotopes are unstable. The most stable isotope, 232Th, has a half-life of 14.05 billion years, or about the age of the universe; it decays very slowly via alpha decay, starting a decay chain named the thorium series that ends at stable 208 Pb. On Earth, thorium and uranium are the only elements with no stable or nearly-stable isotopes that still occur naturally in large quantities as primordial elements. Thorium is estimated to be over three times as abundant as uranium in the Earth's crust, and is chiefly refined from monazite sa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
|
Uranocene
Uranocene, U(C8H8)2, is an organouranium compound composed of a uranium atom sandwiched between two cyclooctatetraene, cyclooctatetraenide rings. It was one of the first Organoactinide chemistry, organoactinide compounds to be synthesized. It is a green air-sensitive solid that dissolves in organic solvents. Uranocene, a member of the "actinocenes," a group of metallocenes incorporating Chemical element, elements from the actinide series. It is the most studied bisCyclooctatetraene, [8]annulene-metal system, although it has no known practical applications. Synthesis, structure and bonding Uranocene was first described in 1968 by the group of Andrew Streitwieser, when it was prepared by the reaction of dipotassium cyclooctatetraenide and uranium tetrachloride in THF at 0°C: : Uranocene is highly reactive toward oxygen, being pyrophoricity, pyrophoric in air but stable to hydrolysis. The x-ray crystal structure of uranocene was first elucidated by the group of Ken Raymond. Conside ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
 |
Organoactinide Chemistry
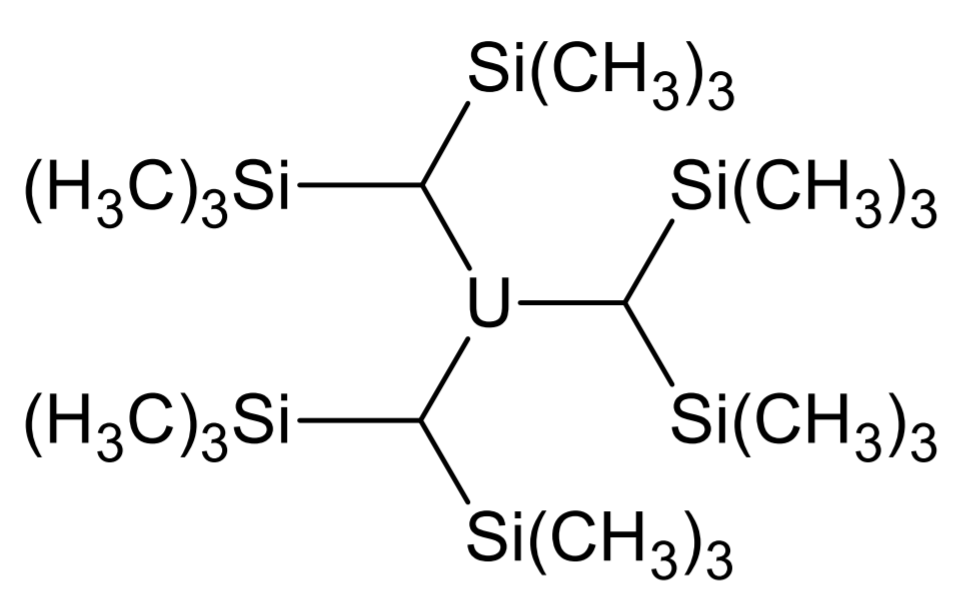
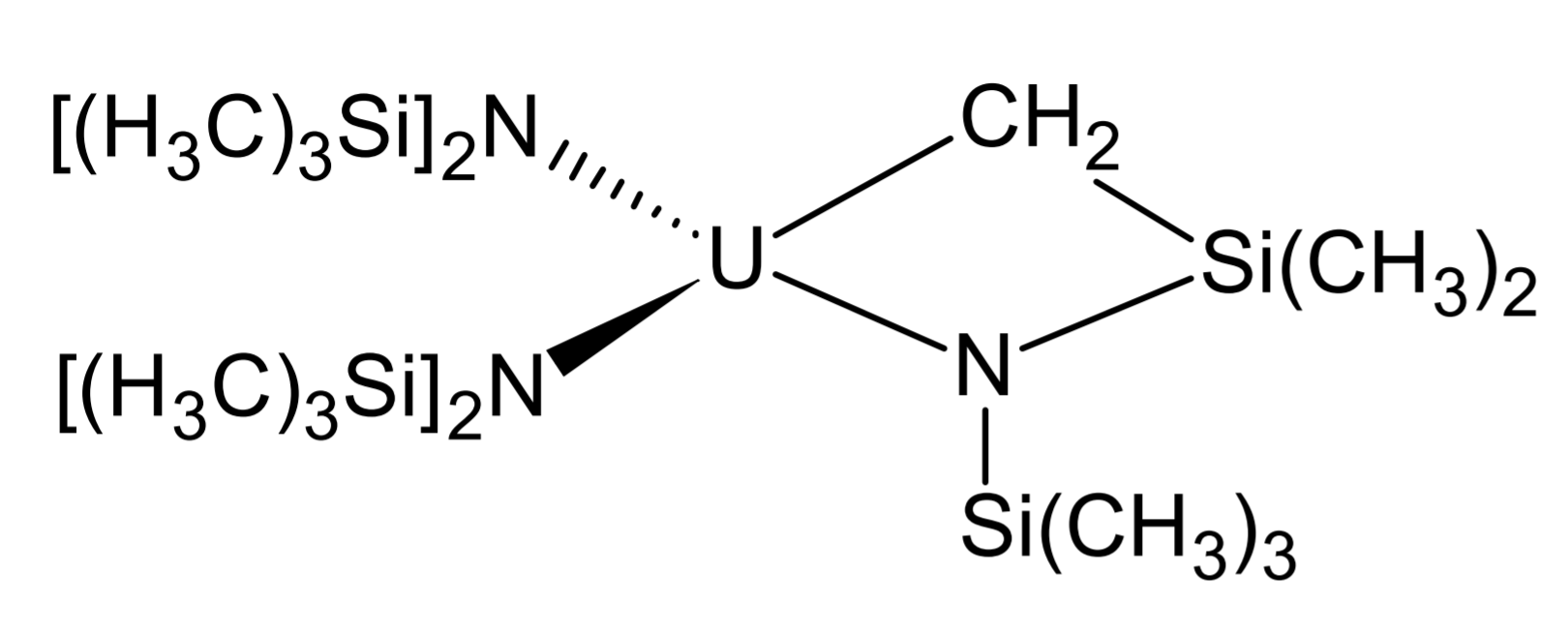
Organoactinide chemistry is the science exploring the properties, structure, and reactivity of organoactinide compounds, which are organometallic compounds containing a carbon to actinide chemical bond. Like most organometallic compounds, the organoactinides are air sensitive and need to be handled using the Air-free technique, appropriate methods. Organometallic complexes with σ-bonding Most common organoactinide complexes involve Pi bond, π-bonding with ligands such as cyclopentadienyl, but there are a few exceptions with Sigma bond, σ-bonding, namely in thorium and uranium chemistry as these are the most easily handleable elements of this group. Alkyl and aryl compounds Attempts to synthesize uranium alkyls were first made during the Manhattan project by Henry Gilman, inspired by the volatility of main group organometallics. However he noticed that these compounds tend to be highly unstable. Marks and Seyam attempted to synthesize them from Uranium tetrachloride, UCl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
Neptunocene
Neptunocene, Np(C8H8)2, is an organoneptunium compound composed of a neptunium atom sandwiched between two cyclooctatetraenide (COT2-) rings. As a solid it has a dark brown/red colour but it appears yellow when dissolved in chlorocarbons, in which it is sparingly soluble. The compound is quite air-sensitive. It was one of the first organoneptunium compounds to be synthesised, and is a member of the actinocene family of actinide-based metallocenes. Structure The sandwich structure of neptunocene has been determined by single crystal XRD. The COT2- rings are found to be planar with 8 equivalent C–C bonds of 1.385 Å length, and sit parallel in an eclipsed conformation. The Np–COT distance (to the ring centroid) is 1.909 Å and the individual Np–C distances are 2.630 Å. Neptunocene assumes a monoclinic crystal structure (''P''21/''n'' space group) which is isomorphous to uranocene and thorocene but not to plutonocene. Synthesis and properties Neptunocene was first syn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
|
Plutonocene
Plutonocene, Pu(C8H8)2, is an organoplutonium compound composed of a plutonium atom sandwiched between two cyclooctatetraenide (COT2-) rings. It is a dark red, very air-sensitive solid that is sparingly soluble in toluene and chlorocarbons. Plutonocene is a member of the actinocene family of metallocenes incorporating actinide elements in the +4 oxidation state. Compared to other actinocenes such as uranocene, plutonocene has been studied to a lesser degree since the 1980s due to the notable radiation hazard posed by the compound. Instead, it has mostly been the subject of theoretical studies relating to the bonding in the molecule. Structure and bonding The compound has been structurally characterised by single crystal XRD. The cyclooctatetraenide rings are eclipsed and assume a planar conformation with 8 equivalent C–C bonds of 1.41 Å length; the molecule possesses a centre of inversion at the position occupied by the plutonium atom. The Pu–COT distance (to the ring ce ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
 |
Organoactinide
Organoactinide chemistry is the science exploring the properties, structure, and reactivity of organoactinide compounds, which are organometallic compounds containing a carbon to actinide chemical bond. Like most organometallic compounds, the organoactinides are air sensitive and need to be handled using the appropriate methods. Organometallic complexes with σ-bonding Most common organoactinide complexes involve π-bonding with ligands such as cyclopentadienyl, but there are a few exceptions with σ-bonding, namely in thorium and uranium chemistry as these are the most easily handleable elements of this group. Alkyl and aryl compounds Attempts to synthesize uranium alkyls were first made during the Manhattan project by Henry Gilman, inspired by the volatility of main group organometallics. However he noticed that these compounds tend to be highly unstable. Marks and Seyam attempted to synthesize them from UCl using organolithium reagents, but these decomposed quickly. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
 |
Lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (element 71) is also sometimes considered a lanthanide, despite being a d-block element and a transition metal. The informal chemical symbol Ln is used in general discussions of lanthanide chemistry to refer to any lanthanide. All but one of the lanthanides are f-block elements, corresponding to the filling of the 4f electron shell. Lutetium is a d-block element (thus also a transition metal), and on this basis its inclusion has been questioned; however, like its congeners scandium and yttrium in group 3, it behaves similarly to the other 14. The term rare-earth element or rare-earth metal is often used to include the stable group 3 elements Sc, Y, and Lu in addition to the 4f elements. All lanthanide elements form trivalent cations, Ln3+, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
Metal–ligand Multiple Bond
In organometallic chemistry, a metal–ligand multiple bond describes the interaction of certain ligands with a metal with a bond order greater than one."Metal–Ligand Multiple Bonds: The Chemistry of Transition Metal Complexes Containing Oxo, Nitrido, Imido, Alkylidene, or Alkylidyne Ligands" W. A. Nugent and J. M. Mayer; Wiley-Interscience, New York, 1988. . Coordination complexes featuring multiply bonded ligands are of both scholarly and practical interest. transition metal carbene complexes catalyze the olefin metathesis reaction. Metal oxo intermediates are pervasive in oxidation catalysis. : As a cautionary note, the classification of a metal–ligand bond as being "multiple" bond order is ambiguous and even arbitrary because bond order is a formalism. Furthermore, the usage of multiple bonding is not uniform. Symmetry arguments suggest that most ligands engage metals via multiple bonds. The term 'metal–ligand multiple bond" is often reserved for ligands of the type and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
|
RSC Advances
''RSC Advances'' is an online-only peer-reviewed scientific journal covering research on all aspects of the chemical sciences. It was established in 2011 and is published by the Royal Society of Chemistry. The current editors-in-chief are Russell Cox ( Leibniz Universität Hannover) and Karen Faulds (University of Strathclyde). In 2014, the journal moved to a very high publication frequency, initially about 100/year (similar to that of '' ChemComm''), but later in the year (and in 2015) turned to even higher frequency—however it did not become a continuous journal. The number of pages published annually had increased dramatically from about 26,500 in 2013 to over 65,000 in 2014, culminating at 116 issues and 115,000+ pages (~1000 pages per issue) in 2016. In late 2016, it was announced that with effect from January 2017, the journal would convert from a subscription based journal to an open access journal. Meanwhile, the journal experienced a cool-down in publication volume, w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
|
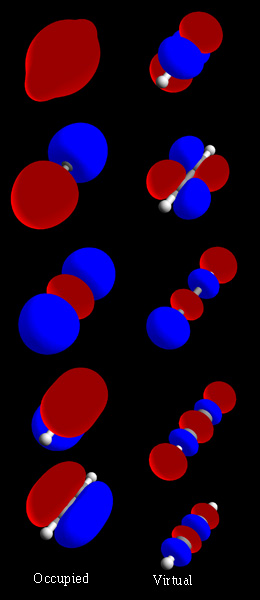 |
Molecular Orbitals
In chemistry, a molecular orbital is a mathematical function describing the location and wave-like behavior of an electron in a molecule. This function can be used to calculate chemical and physical properties such as the probability of finding an electron in any specific region. The terms ''atomic orbital'' and ''molecular orbital'' were introduced by Robert S. Mulliken in 1932 to mean ''one-electron orbital wave functions''. At an elementary level, they are used to describe the ''region'' of space in which a function has a significant amplitude. In an isolated atom, the orbital electrons' location is determined by functions called atomic orbitals. When multiple atoms combine chemically into a molecule by forming a valence chemical bond, the electrons' locations are determined by the molecule as a whole, so the atomic orbitals combine to form molecular orbitals. The electrons from the constituent atoms occupy the molecular orbitals. Mathematically, molecular orbitals are an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
 |
Computational Chemistry
Computational chemistry is a branch of chemistry that uses computer simulations to assist in solving chemical problems. It uses methods of theoretical chemistry incorporated into computer programs to calculate the structures and properties of molecules, groups of molecules, and solids. The importance of this subject stems from the fact that, with the exception of some relatively recent findings related to the hydrogen molecular ion (dihydrogen cation), achieving an accurate quantum mechanical depiction of chemical systems analytically, or in a closed form, is not feasible. The complexity inherent in the many-body problem exacerbates the challenge of providing detailed descriptions of quantum mechanical systems. While computational results normally complement information obtained by chemical experiments, it can occasionally predict unobserved chemical phenomena. Overview Computational chemistry differs from theoretical chemistry, which involves a mathematical description of chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
 |
Chemical Bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds, or some combination of these effects. Chemical bonds are described as having different strengths: there are "strong bonds" or "primary bonds" such as covalent, ionic and metallic bonds, and "weak bonds" or "secondary bonds" such as dipole–dipole interactions, the London dispersion force, and hydrogen bonding. Since opposite electric charges attract, the negatively charged electrons surrounding the nucleus and the positively charged protons within a nucleus attract each other. Electrons shared between two nuclei will be attracted to both of them. "Constructive quantum mechanical wavefunction interference" stabilizes the paired nuclei (see Theories of chemical bonding). Bonded nuclei maintain an optima ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |