|
Thioacetamide
Thioacetamide is an organosulfur compound with the formula C2 H5 N S. This white crystalline solid is soluble in water and serves as a source of sulfide ions in the synthesis of organic and inorganic compounds. It is a prototypical thioamide. Research Thioacetamide is known to induce acute or chronic liver disease (fibrosis and cirrhosis) in the experimental animal model. Its administration in rat induces hepatic encephalopathy, metabolic acidosis, increased levels of transaminases, abnormal coagulation, and centrilobular necrosis, which are the main features of the clinical chronic liver disease so thioacetamide can precisely replicate the initiation and progression of human liver disease in an experimental animal model. Coordination chemistry Thioacetamide is widely used in classical qualitative inorganic analysis as an in situ source for sulfide ions. Thus, treatment of aqueous solutions of many metal cations to a solution of thioacetamide affords the corresponding metal s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioamides
A thioamide (rarely, thionamide, but also known as thiourylenes) is a functional group with the general structure , where are any groups (typically organyl groups or hydrogen). Analogous to amides, thioamides exhibit greater multiple bond character along the C-N bond, resulting in a larger rotational barrier. Synthesis Thioamides are typically prepared by treating amides with phosphorus sulfides such as phosphorus pentasulfide, a reaction first described in the 1870s. An alternative to P2S5 is its more soluble analogue Lawesson's reagent. The Willgerodt-Kindler reaction can give benzylthioamides via an analogous process. These transformations can be seen in the synthesis of tolrestat. : The reaction of nitriles with hydrogen sulfide also affords thioamides: : Imidoyl chlorides react with hydrogen sulfide to produce thioamides. : Reactions A well-known thioamide is thioacetamide, which is used as a source of the sulfide ion. Thioamides are precursors to heterocycles. S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IARC Group 2B Carcinogens
IARC group 2B substances, mixtures and exposure circumstances are those that have been classified as "possibly carcinogenic to humans" by the International Agency for Research on Cancer (IARC) as This category is used when there is level of evidence, limited evidence of carcinogenicity in humans and less than sufficient evidence of carcinogenicity in experimental animals. It may also be used when there is insufficient evidence of carcinogenicity in humans but sufficient evidence in experimental animals. In some cases, an agent, mixture, or exposure circumstance with inadequate evidence of carcinogenicity in humans but limited evidence in experimental animals, combined with supporting evidence from other relevant data, may be included in this group. This list focuses on the hazard linked to the agents. This means that the carcinogenic agents are capable of causing cancer, but this does not take their risk into account, which is the probability of causing a cancer given the level o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioamide
A thioamide (rarely, thionamide, but also known as thiourylenes) is a functional group with the general structure , where are any groups (typically organyl groups or hydrogen). Analogous to amides, thioamides exhibit greater multiple bond character along the C-N bond, resulting in a larger rotational barrier. Synthesis Thioamides are typically prepared by treating amides with phosphorus sulfides such as phosphorus pentasulfide, a reaction first described in the 1870s. An alternative to P2S5 is its more soluble analogue Lawesson's reagent. The Willgerodt-Kindler reaction can give benzylthioamides via an analogous process. These transformations can be seen in the synthesis of tolrestat. : The reaction of nitriles with hydrogen sulfide also affords thioamides: : Imidoyl chlorides react with hydrogen sulfide to produce thioamides. : Reactions A well-known thioamide is thioacetamide, which is used as a source of the sulfide ion. Thioamides are precursors to heterocycles. Such ap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfide
Sulfide (also sulphide in British English) is an inorganic anion of sulfur with the chemical formula S2− or a compound containing one or more S2− ions. Solutions of sulfide salts are corrosive. ''Sulfide'' also refers to large families of inorganic and organic compounds, e.g. lead sulfide and dimethyl sulfide. Hydrogen sulfide (H2S) and bisulfide (HS−) are the conjugate acids of sulfide. Chemical properties The sulfide ion does not exist in aqueous alkaline solutions of Na2S. Instead sulfide converts to hydrosulfide: :S2− + H2O → SH− + OH− Upon treatment with an acid, sulfide salts convert to hydrogen sulfide: :S2− + H+ → SH− :SH− + H+ → H2S Oxidation of sulfide is a complicated process. Depending on the conditions, the oxidation can produce elemental sulfur, polysulfides, polythionates, sulfite, or sulfate. Metal sulfides react with halogens, forming sulfur and metal salts. :8 MgS + 8 I2 → S8 + 8 MgI2 Metal de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartic Acid
Aspartic acid (symbol Asp or D; the ionic form is known as aspartate), is an α-amino acid that is used in the biosynthesis of proteins. The L-isomer of aspartic acid is one of the 22 proteinogenic amino acids, i.e., the building blocks of proteins. D-aspartic acid is one of two D-amino acids commonly found in mammals. Apart from a few rare exceptions, D-aspartic acid is not used for protein synthesis but is incorporated into some peptides and plays a role as a neurotransmitter/ neuromodulator. Like all other amino acids, aspartic acid contains an amino group and a carboxylic acid. Its α-amino group is in the protonated –NH form under physiological conditions, while its α-carboxylic acid group is deprotonated −COO− under physiological conditions. Aspartic acid has an acidic side chain (CH2COOH) which reacts with other amino acids, enzymes and proteins in the body. Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aspartate Transaminase
Aspartate transaminase (AST) or aspartate aminotransferase, also known as AspAT/ASAT/AAT or (serum) glutamic oxaloacetic transaminase (GOT, SGOT), is a pyridoxal phosphate (PLP)-dependent transaminase enzyme () that was first described by Arthur Karmen and colleagues in 1954. AST catalyzes the reversible transfer of an α-amino group between aspartate and glutamate and, as such, is an important enzyme in amino acid metabolism. AST is found in the liver, heart, skeletal muscle, kidneys, brain, red blood cells and gall bladder. Serum AST level, serum ALT (alanine transaminase) level, and their ratio (AST/ALT ratio) are commonly measured clinically as biomarkers for liver health. The tests are part of blood test, blood panels. The biological half-life, half-life of total AST in the circulation approximates 17 hours and, on average, 87 hours for ''mitochondrial'' AST. Aminotransferase is cleared by Liver sinusoidal endothelial cell, sinusoidal cells in the liver. Function Aspartate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
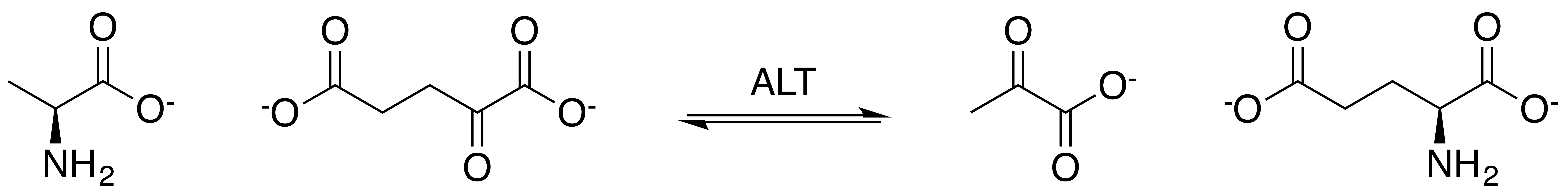
Alanine Transaminase
Alanine aminotransferase (ALT or ALAT), formerly alanine transaminase (ALT), and even earlier referred to as serum glutamate-pyruvate transaminase (GPT) or serum glutamic-pyruvic transaminase (SGPT), is a transaminase enzyme () that was first characterized in the mid-1950s by Arthur Karmen and colleagues. ALT is found in plasma and in various body tissues but is most common in the liver. It catalyzes the two parts of the alanine cycle. Serum ALT level, serum AST ( aspartate transaminase) level, and their ratio ( AST/ALT ratio) are routinely measured clinically as biomarkers for liver health. The half-life of ALT in the circulation approximates 47 hours. Aminotransferase is cleared by sinusoidal cells in the liver. Function ALT catalyzes the transfer of an amino group from L-alanine to α-ketoglutarate, the products of this reversible transamination reaction being pyruvate and L-glutamate. :L-alanine + α-ketoglutarate ⇌ pyruvate + L-glutamate ALT (and all aminotran ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carcinogen
A carcinogen () is any agent that promotes the development of cancer. Carcinogens can include synthetic chemicals, naturally occurring substances, physical agents such as ionizing and non-ionizing radiation, and biologic agents such as viruses and bacteria. Most carcinogens act by creating mutations in DNA that disrupt a cell's normal processes for regulating growth, leading to uncontrolled cellular proliferation. This occurs when the cell's DNA repair processes fail to identify DNA damage allowing the defect to be passed down to daughter cells. The damage accumulates over time. This is typically a multi-step process during which the regulatory mechanisms within the cell are gradually dismantled allowing for unchecked cellular division. The specific mechanisms for carcinogenic activity is unique to each agent and cell type. Carcinogens can be broadly categorized, however, as activation-dependent and activation-independent which relate to the agent's ability to engage dir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphorus Pentasulfide
Phosphorus pentasulfide is the inorganic compound with the formula (empirical) or ( molecular). This yellow solid is the one of two phosphorus sulfides of commercial value. Samples often appear greenish-gray due to impurities. It is soluble in carbon disulfide but reacts with many other solvents such as alcohols, DMSO, and DMF. Structure and synthesis Its tetrahedral molecular structure is similar to that of adamantane and almost identical to the structure of phosphorus pentoxide. Phosphorus pentasulfide is obtained by the reaction of liquid white phosphorus () with sulfur above 300 °C. The first synthesis of by Berzelius in 1843 was by this method. Alternatively, can be formed by reacting elemental sulfur or pyrite, , with ferrophosphorus, a crude form of (a byproduct of white phosphorus () production from phosphate rock): : : Applications Approximately 150,000 tons of are produced annually. The compound is mainly converted to other derivatives for us ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HSAB
HSAB is an acronym for "hard and soft (Lewis) acids and bases". HSAB is widely used in chemistry for explaining the stability of compounds, reaction mechanisms and pathways. It assigns the terms 'hard' or 'soft', and 'acid' or 'base' to chemical species. 'Hard' applies to species which are small, have high charge states (the charge criterion applies mainly to acids, to a lesser extent to bases), and are weakly polarizable. 'Soft' applies to species which are big, have low charge states and are strongly polarizable. The theory is used in contexts where a qualitative, rather than quantitative, description would help in understanding the predominant factors which drive chemical properties and reactions. This is especially so in transition metal chemistry, where numerous experiments have been done to determine the relative ordering of ligands and transition metal ions in terms of their hardness and softness. HSAB theory is also useful in predicting the products of metathesis react ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Qualitative Inorganic Analysis
Classical qualitative inorganic analysis is a method of analytical chemistry which seeks to find the elemental composition of inorganic compounds. It is mainly focused on detecting ions in an aqueous solution, therefore materials in other forms may need to be brought to this state before using standard methods. The solution is then treated with various reagents to test for reactions characteristic of certain ions, which may cause color change, precipitation and other visible changes. Qualitative inorganic analysis is that branch or method of analytical chemistry which seeks to establish the elemental composition of inorganic compounds through various reagents. Physical appearance of some inorganic compounds Detecting cations According to their properties, cations are usually classified into six groups. Each group has a common reagent which can be used to separate them from the solution. To obtain meaningful results, the separation must be done in the sequence specified bel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Union Of Pure And Applied Chemistry
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national chemistry societies, national academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organizations. IUPAC's Inter-divisional Committee ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |