|
Thioamides
A thioamide (rarely, thionamide, but also known as thiourylenes) is a functional group with the general structure , where are any groups (typically organyl groups or hydrogen). Analogous to amides, thioamides exhibit greater multiple bond character along the C-N bond, resulting in a larger rotational barrier. Synthesis Thioamides are typically prepared by treating amides with phosphorus sulfides such as phosphorus pentasulfide, a reaction first described in the 1870s. An alternative to P2S5 is its more soluble analogue Lawesson's reagent. The Willgerodt-Kindler reaction can give benzylthioamides via an analogous process. These transformations can be seen in the synthesis of tolrestat. : The reaction of nitriles with hydrogen sulfide also affords thioamides: : Imidoyl chlorides react with hydrogen sulfide to produce thioamides. : Reactions A well-known thioamide is thioacetamide, which is used as a source of the sulfide ion. Thioamides are precursors to heterocycles. S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioamide
A thioamide (rarely, thionamide, but also known as thiourylenes) is a functional group with the general structure , where are any groups (typically organyl groups or hydrogen). Analogous to amides, thioamides exhibit greater multiple bond character along the C-N bond, resulting in a larger rotational barrier. Synthesis Thioamides are typically prepared by treating amides with phosphorus sulfides such as phosphorus pentasulfide, a reaction first described in the 1870s. An alternative to P2S5 is its more soluble analogue Lawesson's reagent. The Willgerodt-Kindler reaction can give benzylthioamides via an analogous process. These transformations can be seen in the synthesis of tolrestat. : The reaction of nitriles with hydrogen sulfide also affords thioamides: : Imidoyl chlorides react with hydrogen sulfide to produce thioamides. : Reactions A well-known thioamide is thioacetamide, which is used as a source of the sulfide ion. Thioamides are precursors to heterocycles. Such ap ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioamides
A thioamide (rarely, thionamide, but also known as thiourylenes) is a functional group with the general structure , where are any groups (typically organyl groups or hydrogen). Analogous to amides, thioamides exhibit greater multiple bond character along the C-N bond, resulting in a larger rotational barrier. Synthesis Thioamides are typically prepared by treating amides with phosphorus sulfides such as phosphorus pentasulfide, a reaction first described in the 1870s. An alternative to P2S5 is its more soluble analogue Lawesson's reagent. The Willgerodt-Kindler reaction can give benzylthioamides via an analogous process. These transformations can be seen in the synthesis of tolrestat. : The reaction of nitriles with hydrogen sulfide also affords thioamides: : Imidoyl chlorides react with hydrogen sulfide to produce thioamides. : Reactions A well-known thioamide is thioacetamide, which is used as a source of the sulfide ion. Thioamides are precursors to heterocycles. S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tolrestat
Tolrestat ( INN; AY-27773) is an aldose reductase inhibitor which was approved for the control of certain diabetic complications. While it was approved for marketed in several countries, it failed a Phase III trial in the U.S. due to toxicity and never received FDA approval. It was discontinued by Wyeth Wyeth Pharmaceuticals Inc. was a pharmaceutical company until it was purchased by Pfizer in 2009. The company was founded in Philadelphia, Pennsylvania, in 1860 as John Wyeth and Brother. Its headquarters moved to Collegeville, Pennsylvania, a ... in 1997 because of the risk of severe liver toxicity and death. It was sold under the tradename Alredase. References Aldose reductase inhibitors Acetic acids Hepatotoxins Naphthol ethers Trifluoromethyl compounds Thioamides Withdrawn drugs {{gastrointestinal-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioacetamide
Thioacetamide is an organosulfur compound with the formula C2 H5 N S. This white crystalline solid is soluble in water and serves as a source of sulfide ions in the synthesis of organic and inorganic compounds. It is a prototypical thioamide. Research Thioacetamide is known to induce acute or chronic liver disease (fibrosis and cirrhosis) in the experimental animal model. Its administration in rat induces hepatic encephalopathy, metabolic acidosis, increased levels of transaminases, abnormal coagulation, and centrilobular necrosis, which are the main features of the clinical chronic liver disease so thioacetamide can precisely replicate the initiation and progression of human liver disease in an experimental animal model. Coordination chemistry Thioacetamide is widely used in classical qualitative inorganic analysis as an in situ source for sulfide ions. Thus, treatment of aqueous solutions of many metal cations to a solution of thioacetamide affords the corresponding metal s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tolrestat Synthesis
Tolrestat ( INN; AY-27773) is an aldose reductase inhibitor which was approved for the control of certain diabetic complications. While it was approved for marketed in several countries, it failed a Phase III trial in the U.S. due to toxicity and never received FDA approval. It was discontinued by Wyeth Wyeth Pharmaceuticals Inc. was a pharmaceutical company until it was purchased by Pfizer in 2009. The company was founded in Philadelphia, Pennsylvania, in 1860 as John Wyeth and Brother. Its headquarters moved to Collegeville, Pennsylvania, a ... in 1997 because of the risk of severe liver toxicity and death. It was sold under the tradename Alredase. References Aldose reductase inhibitors Acetic acids Hepatotoxins Naphthol ethers Trifluoromethyl compounds Thioamides Withdrawn drugs {{gastrointestinal-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thioketone
In organic chemistry, thioketones (; also known as thiones or thiocarbonyls) are organosulfur compounds related to conventional ketones in which the oxygen has been replaced by a sulfur. Instead of a structure of , thioketones have the structure , which is reflected by the prefix " thio-" in the name of the functional group. Thus the simplest thioketone is thioacetone, the sulfur analog of acetone. Unhindered alkylthioketones typically tend to form polymers or rings. Structure and bonding The C=S bond length of thiobenzophenone is 1.63 Å, which is comparable to 1.64 Å, the C=S bond length of thioformaldehyde, measured in the gas phase. Due to steric interactions, the phenyl groups are not coplanar and the dihedral angle SC-CC is 36°. Unhindered dialkylthiones polymerize or oligomerize but thiocamphor is well characterized red solid. Consistent with the double bond rule, most alkyl thioketones are unstable with respect to dimerization.Organosulfur Chemistry I: Topics in Cur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Colloid
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture (although a narrower sense of the word '' suspension'' is distinguished from colloids by larger particle size). A colloid has a dispersed phase (the suspended particles) and a continuous phase (the medium of suspension). The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre. Some colloids are translucent because of the Tyndall effect, which is the scattering of light by particles in the colloid. Other colloids may be opaque or have a slight color. Colloidal suspensions are the subject of interface and colloid science. This field of study began in 1845 by Franc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thyroxine
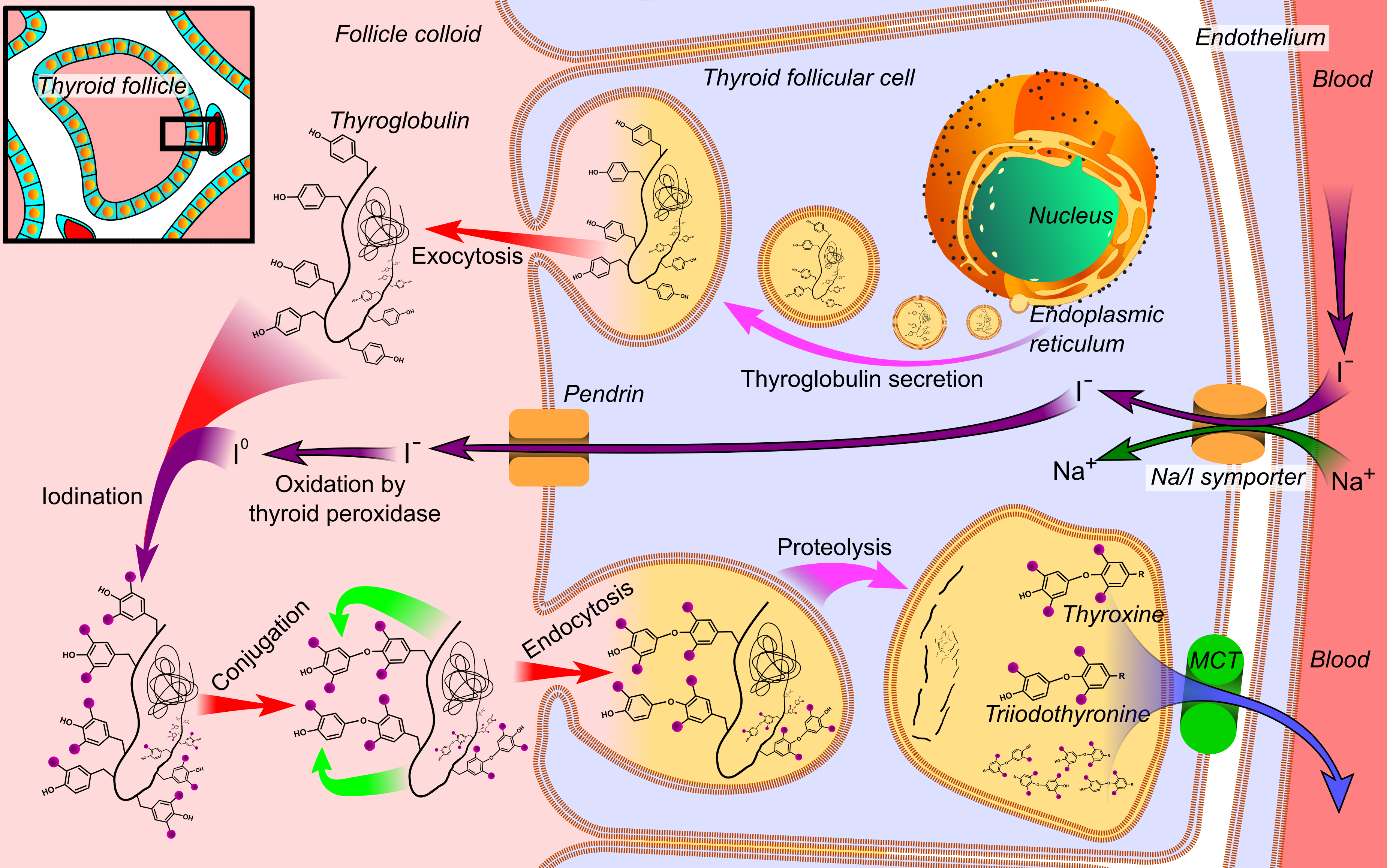
Thyroxine, also known as T4, is a hormone produced by the thyroid gland. It is the primary form of thyroid hormone found in the blood and acts as a prohormone of the more active thyroid hormone, triiodothyronine (T3). Thyroxine and its active metabolites are essential for regulating metabolic rate, supporting heart and muscle function, promoting brain development, and maintaining bone health. Regulation Thyroxine has a half-life of approximately one week and hence maintains relatively stable blood levels. Its production and release are controlled through a complex feedback loop involving the hypothalamus, pituitary gland, and thyroid gland. This regulatory system ensures that optimal hormone levels are maintained. Biosynthesis Thyroxine biosynthesis is a multi-step process that occurs in follicular cell within the thyroid gland. The synthesis of thyroxine requires adequate iodine supply and appropriate hormonal control. The process begins with the active uptake o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triiodothyronine
Triiodothyronine, also known as T3, is a thyroid hormone. It affects almost every physiological process in the body, including growth and development, metabolism, body temperature, and heart rate. Production of T3 and its prohormone thyroxine (T4) is activated by thyroid-stimulating hormone (TSH), which is released from the anterior pituitary gland. This pathway is part of a closed-loop feedback process: Elevated concentrations of T3, and T4 in the blood plasma inhibit the production of TSH in the anterior pituitary gland. As concentrations of these hormones decrease, the anterior pituitary gland increases production of TSH, and by these processes, a feedback control system stabilizes the level of thyroid hormones in the bloodstream. At the cellular level, T3 is the body's more active and potent thyroid hormone. T3 helps deliver oxygen and energy to all of the body's cells, its effects on target tissues being roughly four times more potent than those of T4. Of the thyroid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thyroid
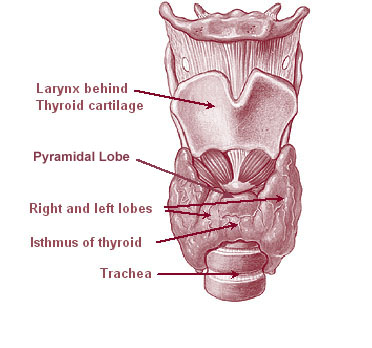
The thyroid, or thyroid gland, is an endocrine gland in vertebrates. In humans, it is a butterfly-shaped gland located in the neck below the Adam's apple. It consists of two connected lobes. The lower two thirds of the lobes are connected by a thin band of tissue called the isthmus (: isthmi). Microscopically, the functional unit of the thyroid gland is the spherical thyroid follicle, lined with follicular cells (thyrocytes), and occasional parafollicular cells that surround a lumen containing colloid. The thyroid gland secretes three hormones: the two thyroid hormones triiodothyronine (T3) and thyroxine (T4)and a peptide hormone, calcitonin. The thyroid hormones influence the metabolic rate and protein synthesis and growth and development in children. Calcitonin plays a role in calcium homeostasis. Secretion of the two thyroid hormones is regulated by thyroid-stimulating hormone (TSH), which is secreted from the anterior pituitary gland. TSH is regulated by thy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thyroid Peroxidase
Thyroid peroxidase, also called thyroperoxidase (TPO), thyroid specific peroxidase or iodide peroxidase, is an enzyme expressed mainly in the thyroid where it is secreted into colloid. Thyroid peroxidase oxidizes iodide ions to form iodine atoms for addition onto tyrosine residues on thyroglobulin for the production of thyroxine (T4) or triiodothyronine (T3), the thyroid hormones. In humans, thyroperoxidase is encoded by the ''TPO'' gene. Function Inorganic iodine enters the body primarily as iodide, I−. After entering the thyroid follicle (or thyroid follicular cell) via a Na+/I− symporter (NIS) on the basolateral side, iodide is shuttled across the apical membrane into the colloid via pendrin after which thyroid peroxidase oxidizes iodide to atomic iodine (I) or iodinium (I+). The chemical reactions catalyzed by thyroid peroxidase occur on the outer apical membrane surface and are mediated by hydrogen peroxide. The "organification of iodine", the incorporation of iodine ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation. By definition, when a medication is administered intravenously, its bioavailability is 100%. However, when a medication is administered via routes other than intravenous, its bioavailability is lower due to intestinal epithelium absorption and first-pass metabolism. Thereby, mathematically, bioavailability equals the ratio of comparing the area under the plasma drug concentration curve versus time (AUC) for the extravascular formulation to the AUC for the intravascular formulation. AUC is used because AUC is proportional to the dose that has entered the systemic circulation. Bioavailability of a drug is an average value; to take population variability into account, deviation range is shown as ±. To ensure that the drug taker who has poor absorption is dosed appropriately, the bottom value of the deviation range is employed ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |