|
Tetradehydrodianthracene
Pyramidal alkenes are alkenes in which the two carbon atoms making up the double bond are not coplanar with their four substituents. This deformation results from geometric constraints. Pyramidal alkenes are of interest because much can be learned from them about the nature of chemical bonding. Energetics Twisting to a 90° dihedral angle between two of the groups on the carbons requires less energy than the strength of a pi bond, and the bond still holds. The carbons of the double bond become pyramidal, which allows preserving some p orbital alignment—and hence pi bonding. The other two attached groups remain at a larger dihedral angle. This contradicts a common textbook assertion that the two carbons retain their planar nature when twisting, in which case the p orbitals would rotate enough away from each other to be unable to sustain a pi bond. In a 90°-twisted alkene, the p orbitals are only misaligned by 42° and the strain energy is only around 40 kcal/mol. In contrast, a fu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Möbius Aromaticity
In organic chemistry, Möbius aromaticity is a special type of aromaticity believed to exist in a number of organic molecules. In terms of molecular orbital theory these compounds have in common a monocyclic array of molecular orbitals in which there is an odd number of out-of-phase overlaps, the opposite pattern compared to the aromatic character in Hückel's rule, Hückel systems. The nodal plane of the orbitals, viewed as a ribbon, is a Möbius strip, rather than a cylinder, hence the name. The pattern of orbital energies is given by a rotated Möbius–Hückel concept, Frost circle (with the edge of the polygon on the bottom instead of a vertex), so systems with 4''n'' electrons are aromatic, while those with 4''n'' + 2 electrons are anti-aromatic/non-aromatic. Due to the incrementally twisted nature of the orbitals of a Möbius aromatic system, stable Möbius aromatic molecules need to contain at least 8 electrons, although 4-electron Möbius aromatic transition states are w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins. The International Union of Pure and Applied Chemistry (IUPAC) Preferred IUPAC name, recommends using the name "alkene" only for Open-chain compound, acyclic hydrocarbons with just one double bond; alkadiene, alkatriene, etc., or polyene for acyclic hydrocarbons with two or more double bonds; cycloalkene, cycloalkadiene, etc. for Cyclic compound, cyclic ones; and "olefin" for the general class – cyclic or acyclic, with one or more double bonds. Acyclic alkenes, with only one double bond and no other functional groups (also known as mono-enes) form a homologous series of hydrocarbons with the general formula with ''n'' being a >1 natural number (which is two hydrogens less than the corresponding alkane). When ''n'' is four or more, isomers are possible, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and is commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercial or biological significance, but more complex derivatives are important in biology and biotechnology. Structure The bond angles between carbon atoms are significantly strained and as such have lower bond energies than related linear or unstrained hydrocarbons, e.g. butane or cyclohexane. As such, cyclobutane is unstable above about 500 °C. The four carbon atoms in cyclobutane are not coplanar; instead, the ring typically adopts a folded or "puckered" conformation. This implies that the C-C-C angle is less than 90°. One of the carbon atoms makes a 25° angle with the plane formed by the other three carbons. In this way, some of the eclipsing interactions are reduced. The conformation is also known as a "butterfly". Equivalent ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Tert-butoxide
Potassium ''tert''-butoxide (or potassium ''t''-butoxide) is a chemical compound with the formula CH3)3COKsub>''n'' (abbr. KOtBu). This colourless solid is a strong base (pKa of conjugate acid is 17 in H2O), which is useful in organic synthesis. The compound is often depicted as a salt, and it often behaves as such, but its ionization depends on the solvent. Preparation Potassium ''t''-butoxide is commercially available as a solution and as a solid, but it is often generated ''in situ'' for laboratory use because samples are so moisture- sensitive and older samples are often of low purity. It is prepared by the reaction of dry ''tert''-butyl alcohol with potassium metal. The solid is obtained by evaporating these solutions followed by heating the solid. The solid can be purified by sublimation. Structure It crystallizes as a tetrameric cubane-type cluster. It crystallises from tetrahydrofuran/pentane at −20 °C as BuOK·tBuOHsub>∞, which consists of straig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cyclophane
In organic chemistry, a cyclophane is a hydrocarbon consisting of an aromatic unit (typically a benzene ring) and a Catenation, chain that forms a bridge (chemical), bridge between two non-adjacent positions of the aromatic ring. More complex derivatives with multiple aromatic units and bridges forming cagelike structures are also known. Cyclophanes are well-studied examples of Strain (chemistry), strained organic compounds. [n]-Cyclophanes Structures Paracyclophanes adopt the boat conformation normally observed in cyclohexanes. Smaller value of n lead to greater distortions. X-ray crystallography on '[6]paracyclophane' shows that the aromatic bridgehead carbon atom makes an angle of 20.5° with the plane. The benzyl carbons deviate by another 20.2°. The carbon-to-carbon bond length alternation has increased from 0 for benzene to 39 picometer, pm. Despite their distorted structures, cyclophanes retain their aromaticity, as determined by UV-vis spectroscopy. Reactivity With re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Through Space Bond
Adpositions are a class of words used to express spatial or temporal relations (''in, under, towards, behind, ago'', etc.) or mark various semantic roles (''of, for''). The most common adpositions are prepositions (which precede their complement) and postpositions (which follow their complement). An adposition typically combines with a noun phrase, this being called its complement, or sometimes object. English generally has prepositions rather than postpositions – words such as ''in, under'' and ''of'' precede their objects, such as "in England", "under the table", "of Jane" – although there are a few exceptions including ''ago'' and ''notwithstanding'', as in "three days ago" and "financial limitations notwithstanding". Some languages that use a different word order have postpositions instead (like Turkic languages) or have both types (like Finnish). The phrase formed by an adposition together with its complement is called an adpositional phrase (or prepositional phrase, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
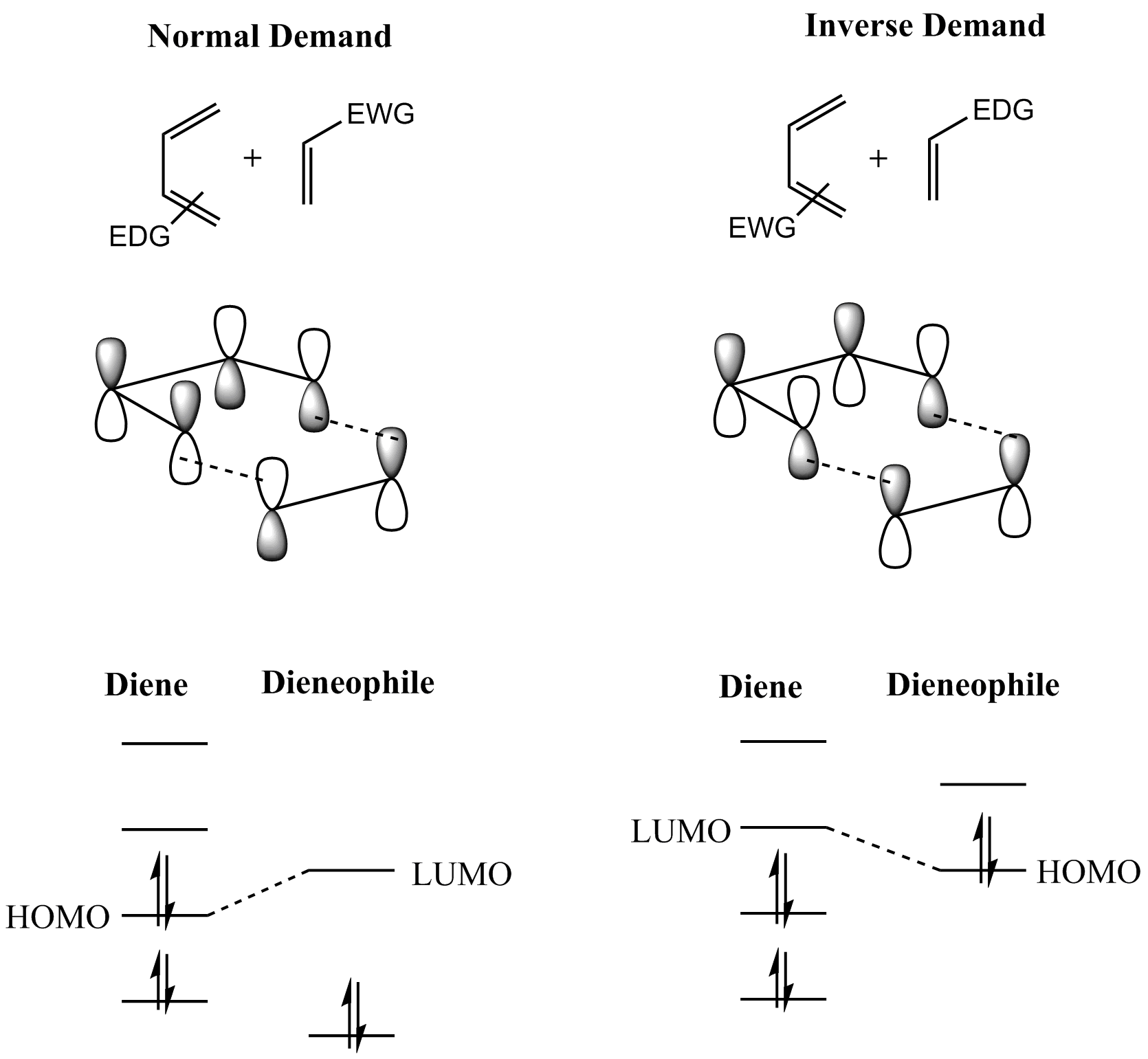
Diels–Alder Reaction
In organic chemistry, the Diels–Alder reaction is a chemical reaction between a Conjugated system, conjugated diene and a substituted alkene, commonly termed the Diels–Alder reaction#The dienophile, dienophile, to form a substituted cyclohexene derivative. It is the prototypical example of a pericyclic reaction with a concerted reaction, concerted mechanism. More specifically, it is classified as a thermally allowed [4+2] cycloaddition with Woodward–Hoffmann rules, Woodward–Hoffmann symbol [π4s + π2s]. It was first described by Otto Diels and Kurt Alder in 1928. For the discovery of this reaction, they were awarded the Nobel Prize in Chemistry in 1950. Through the simultaneous construction of two new carbon–carbon bonds, the Diels–Alder reaction provides a reliable way to form six-membered rings with good control over the regio- and stereochemical outcomes. Consequently, it has served as a powerful and widely applied tool for the introduction of chemical complexity in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrogen Bromide
Hydrogen bromide is the inorganic compound with the formula . It is a hydrogen halide consisting of hydrogen and bromine. A colorless gas, it dissolves in water, forming hydrobromic acid, which is saturated at 68.85% HBr by weight at room temperature. Aqueous solutions that are 47.6% HBr by mass form a constant-boiling azeotrope mixture that boils at . Boiling less concentrated solutions releases H2O until the constant-boiling mixture composition is reached. Hydrogen bromide, and its aqueous solution, hydrobromic acid, are commonly used reagents in the preparation of bromide compounds. Reactions Organic chemistry Hydrogen bromide and hydrobromic acid are important reagents in the production of organobromine compounds.Greenwood, N. N.; Earnshaw, A. Chemistry of the Elements; Butterworth-Heineman: Oxford, Great Britain; 1997; pp. 809–812.Vollhardt, K. P. C.; Neil E. Schore, Schore, N. E. Organic Chemistry: Structure and Function; 4th Ed.; W. H. Freeman and Company: New York, N ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elimination Reaction
An elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism is known as the E2 reaction, and the two-step mechanism is known as the E1 reaction. The numbers refer not to the number of steps in the mechanism, but rather to the kinetics of the reaction: E2 is bimolecular (second-order) while E1 is unimolecular (first-order). In cases where the molecule is able to stabilize an anion but possesses a poor leaving group, a third type of reaction, E1cB-elimination reaction, E1CB, exists. Finally, the pyrolysis of xanthate and acetate esters proceed through an "internal" elimination mechanism, the Ei mechanism, Ei mechanism. E2 mechanism The E2 mechanism, where E2 stands for bimolecular elimination, involves a one-step mechanism in which ''carbon-hydrogen'' and ''carbon-halogen'' bonds break to form a double bond (''C=C molecular geometry, Pi bond''). The specifics of the re ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anthracene
Anthracene is a solid polycyclic aromatic hydrocarbon (PAH) of formula C14H10, consisting of three fused benzene rings. It is a component of coal tar. Anthracene is used in the production of the red dye alizarin and other dyes, as a scintillator to detect high energy particles, as production of pharmaceutical drugs. Anthracene is colorless but exhibits a blue (400–500 nm peak) fluorescence under ultraviolet radiation. History and etymology Crude anthracene (with a melting point of only 180°) was discovered in 1832 by Jean-Baptiste Dumas and Auguste Laurent who crystalized it from a fraction of coal tar later known as "anthracene oil". Since their (inaccurate) measurements showed the proportions of carbon and hydrogen of it to be the same as in naphthalene, Laurent called it ''paranaphtaline'' in his 1835 publication of the discovery, which is translated to English as paranaphthalene. Two years later, however, he decided to rename the compound to its modern name d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |