|
Tetraborate
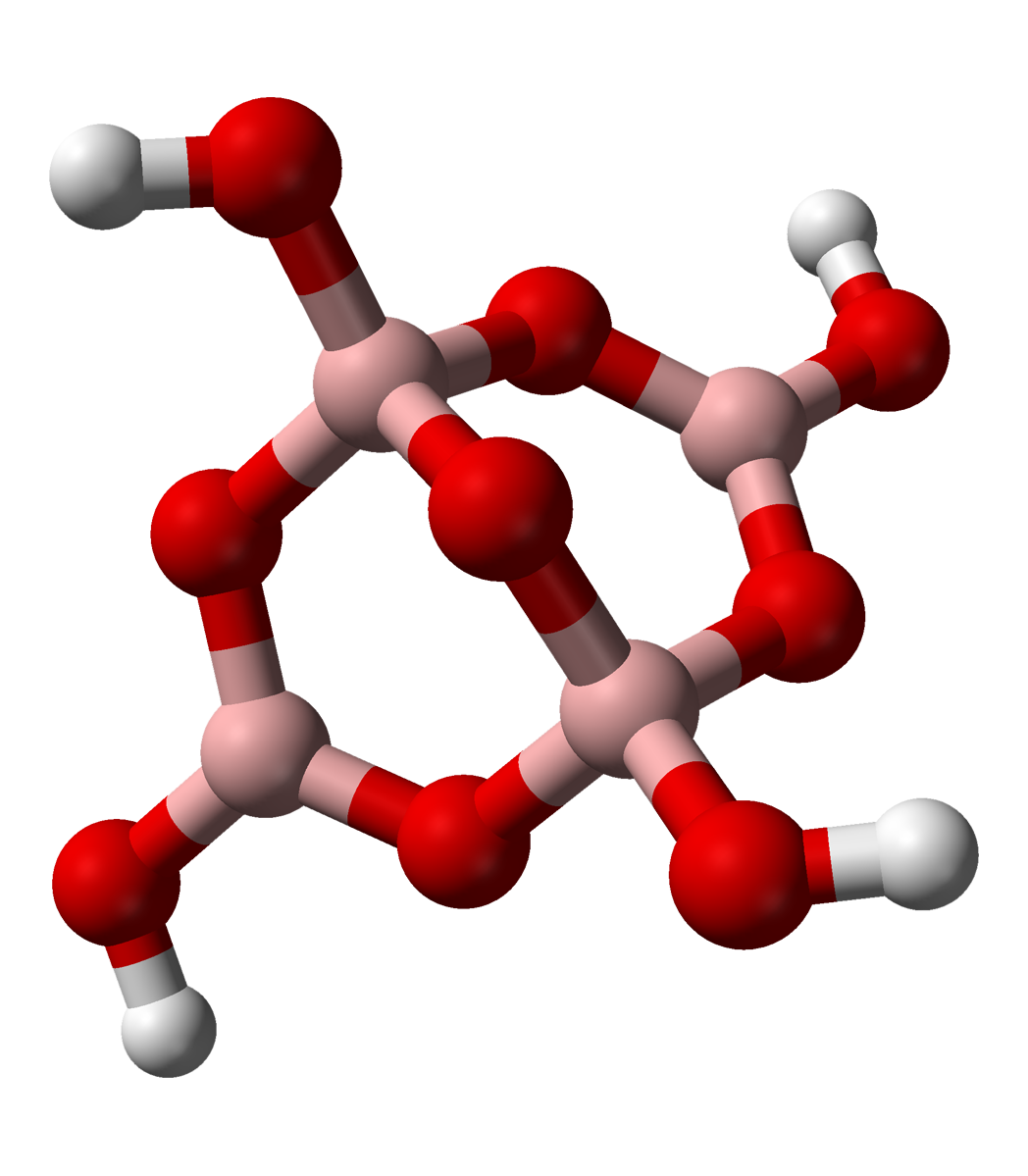
In chemistry, tetraborate or pyroborate is an anion (negative ion) with formula ; or a salt containing that anion, such as sodium tetraborate, . It is one of the boron oxoacids, that is, a borate. The name is also applied to the hydrated ion as present in borax The ion occurs in boric acid solutions at neutral pH, being formed by condensation of orthoborate and tetrahydroxyborate anions: : 2 B(OH)3 + 2 ⇌ + 5 H2O The tetraborate anion (tetramer) includes two tetrahedral and two trigonal boron atoms symmetrically assembled in a fused bicyclic structure. The two tetrahedral boron atoms are linked together by a common oxygen atom, and each also bears a negative net charge brought by the supplementary OH− groups laterally attached to them. This intricate molecular anion also exhibits three rings: two fused distorted hexagonal (boroxole) rings and one distorted octagonal ring. Each ring is made of a succession of alternate boron and oxygen atoms. Boroxole rings are a very c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borates
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt (chemistry), salt of such anions, such as sodium metaborate, and borax . The name also refers to esters of such anions, such as trimethyl borate . Natural occurrence Borate ions occur, alone or with other anions, in many borate mineral, borate and borosilicate minerals such as borax, boracite, ulexite (boronatrocalcite) and colemanite. Borates also occur in seawater, contributing to the absorption of low-frequency sound in seawater. Common borate salts include sodium metaborate (NaBO2) and borax. Borax is soluble in water, so mineral deposits only occur in places with very low rainfall. Extensive deposits were found in Death Valley and shipped with twenty-mule teams from 1883 to 1889. In 1925, deposits were found at Boron, California, Boron, California on the edge of the Mojave Desert. The Atacama Desert in Chile also contains mineab ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borate
A borate is any of a range of boron oxyanions, anions containing boron and oxygen, such as orthoborate , metaborate , or tetraborate ; or any salt of such anions, such as sodium metaborate, and borax . The name also refers to esters of such anions, such as trimethyl borate . Natural occurrence Borate ions occur, alone or with other anions, in many borate and borosilicate minerals such as borax, boracite, ulexite (boronatrocalcite) and colemanite. Borates also occur in seawater, contributing to the absorption of low-frequency sound in seawater. Common borate salts include sodium metaborate (NaBO2) and borax. Borax is soluble in water, so mineral deposits only occur in places with very low rainfall. Extensive deposits were found in Death Valley and shipped with twenty-mule teams from 1883 to 1889. In 1925, deposits were found at Boron, California on the edge of the Mojave Desert. The Atacama Desert in Chile also contains mineable borate concentrations. Borates ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron Heterocycles
Boron is a chemical element; it has Chemical symbol, symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its Amorphous solid, amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral borax, sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovas and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Crust (geology), Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three valence electrons for forming covalent bonds, resulting in many compounds such as boric acid, the mineral sodium borate, and the ultra-hard crystals of boron carbide and boron nitride. Boron is synthesized entirely by cosmic ray spallation and supernovas and not by stellar nucleosynthesis, so it is a low-abundance element in the Solar System and in the Earth's crust. It constitutes about 0.001 percent by weight of Earth's crust. It is concentrated on Earth by the water-solubility of its more common naturally occurring compounds, the borate minerals. These are mined industrially as evaporites, such as borax and kernite. The largest known deposits are in Turkey, the largest producer of boron minerals. Elemental boron is found in smal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Boric Acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen orthoborate, trihydroxidoboron or boracic acid. It is usually encountered as colorless crystals or a white powder, that dissolves in water, and occurs in nature as the mineral sassolite. It is a weak acid that yields various borate anions and salt (chemistry), salts, and can react with Alcohol (chemistry), alcohols to form borate esters. Boric acid is often used as an antiseptic, insecticide, flame retardant, neutron absorber, or precursor to other boron compounds. The term "boric acid" is also used generically for any oxyacid of boron, such as metaboric acid and tetraboric acid . History Orthoboric acid was first prepared by Wilhelm Homberg (1652–1715) from borax, by the action of mineral acids, and was given the name ("sedative salt of Homberg"). However, boric acid and borates have been used since the time of the ancient Greece, anc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxoacid
An oxyacid, oxoacid, or ternary acid is an acid that contains oxygen. Specifically, it is a compound that contains hydrogen, oxygen, and at least one other element, with at least one hydrogen atom bonded to oxygen that can dissociate to produce the H+ cation and the anion of the acid. Description Under Lavoisier's original theory, all acids contained oxygen, which was named from . It was later discovered that some acids, notably hydrochloric acid, did not contain oxygen and so acids were divided into oxo-acids and these new hydroacids. All oxyacids have the acidic hydrogen bound to an oxygen atom, so bond strength (length) is not a factor, as it is with binary nonmetal hydrides. Rather, the electronegativity of the central atom and the number of oxygen atoms determine oxyacid acidity. For oxyacids with the same central atom, acid strength increases with the number of oxygen atoms attached to it. With the same number of oxygen atoms attached to it, acid strength increases with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Tetraborate
The BORAX Experiments were a series of safety experiments on boiling water reactor, boiling water nuclear reactors conducted by Argonne National Laboratory in the 1950s and 1960s at the National Reactor Testing Station in eastern Idaho.Light Water Reactor Technology Development Argonne National Laboratory They were performed using the five BORAX reactors that were designed and built by Argonne. BORAX-III was the first nuclear reactor to supply electrical power to the North American power transmission grid, grid in the United States in 1955. Evolution of BORAX This series of tests began in 1952 with the construction of the BORAX-I nuclear reactor. BORAX-I experiment proved that a reactor using direct boiling water reactor, boiling of water would be practical, ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Borax
The BORAX Experiments were a series of safety experiments on boiling water nuclear reactors conducted by Argonne National Laboratory in the 1950s and 1960s at the National Reactor Testing Station in eastern Idaho.Light Water Reactor Technology Development Argonne National Laboratory They were performed using the five BORAX reactors that were designed and built by Argonne. BORAX-III was the first nuclear reactor to supply electrical power to the grid in the United States in 1955. Evolution of BORAX This series of tests began in 1952 with the construction of the BORAX-I |
Chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during chemical reaction, reactions with other chemical substance, substances. Chemistry also addresses the nature of chemical bonds in chemical compounds. In the scope of its subject, chemistry occupies an intermediate position between physics and biology. It is sometimes called the central science because it provides a foundation for understanding both Basic research, basic and Applied science, applied scientific disciplines at a fundamental level. For example, chemistry explains aspects of plant growth (botany), the formation of igneous rocks (geology), how atmospheric ozone is formed and how environmental pollutants are degraded (ecology), the prop ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthoborate
In inorganic chemistry, an orthoborate is a Polyatomic ion, polyatomic anion with formula or a salt (chemistry), salt containing the anion; such as trisodium orthoborate . It is one of several boron oxyanions, or borates. The name is also used in organic chemistry for the Valence (chemistry), trivalent functional group , or any compound (ester) that contains it, such as triethyl orthoborate . Structure The orthoborate ion is known in the solid state, for example, in calcium orthoborate , where it adopts a nearly trigonal planar structure. It is a structural analogue of the carbonate anion , with which it is isoelectronic. Simple bonding theories point to the trigonal planar structure. In terms of valence bond theory, the bonds are formed by using Orbital hybridisation#sp2, sp2 hybrid orbitals on boron. Some compounds termed orthoborates do not necessarily contain the trigonal planar ion. For example, gadolinium orthoborate contains the planar ion only a high temperatures; ot ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tetrahydroxyborate
Tetrahydroxyborate is an inorganic anion with the chemical formula or . It contributes no colour to tetrahydroxyborate salts. It is found in the mineral hexahydroborite, , originally formulated . It is one of the boron oxoanions, and acts as a weak base. The systematic names are tetrahydroxyboranuide (substitutive) and tetrahydroxidoborate(1−) (additive). It can be viewed as the conjugate base of boric acid. Structure Tetrahydroxyborate has a symmetric tetrahedral geometry, isoelectronic with the hypothetical compound orthocarbonic acid (). Chemical properties Basicity Tetrahydroxyborate acts as a weak Brønsted–Lowry base because it can assimilate a proton (), yielding boric acid with release of water: : + + It can also release a hydroxide anion , thus acting as a classical Arrhenius base: : + (pK = 9.14 to the left) Thus, when boric acid is dissolved in pure (neutral) water, most of it will exist as tetrahydroxyborate ions. With diols In aqueous soluti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |