|
Sulphonamide
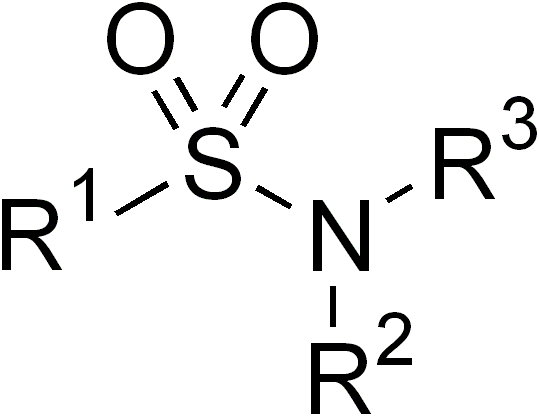
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonamide (medicine)
Sulfonamide is a functional group (a part of a molecule) that is the basis of several groups of drugs, which are called sulphonamides, sulfa drugs or sulpha drugs. The original antibacterial sulfonamides are synthetic (nonantibiotic) antimicrobial agents that contain the sulfonamide group. Some sulfonamides are also devoid of antibacterial activity, e.g., the anticonvulsant sultiame. The sulfonylureas and thiazide diuretics are newer drug groups based upon the antibacterial sulfonamides. Allergies to sulfonamides are common. The overall incidence of adverse drug reactions to sulfa antibiotics is approximately 3%, close to penicillin; hence medications containing sulfonamides are prescribed carefully. Sulfonamide drugs were the first broadly effective antibacterials to be used systemically, and paved the way for the antibiotic revolution in medicine. Function In bacteria, antibacterial sulfonamides act as competitive inhibitors of the enzyme dihydropteroate synthas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sultam
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivative or v ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfonamide
In organic chemistry, the sulfonamide functional group (also spelled sulphonamide) is an organosulfur group with the structure . It consists of a sulfonyl group () connected to an amine group (). Relatively speaking this group is unreactive. Because of the rigidity of the functional group, sulfonamides are typically crystalline; for this reason, the formation of a sulfonamide is a classic method to convert an amine into a crystalline derivative which can be identified by its melting point. Many important drugs contain the sulfonamide group. A sulfonamide (compound) is a chemical compound that contains this group. The general formula is or , where each R is some organic group; for example, "methanesulfonamide" (where R = methane, R' = R" = hydrogen) is . Any sulfonamide can be considered as derived from a sulfonic acid by replacing a hydroxyl group () with an amine group. In medicine, the term "sulfonamide" is sometimes used as a synonym for sulfa drug, a derivat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosulfur
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries. Sulfur shares the chalcogen group with oxygen, selenium, and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium, and carbon–tellurium compounds. A classical chemical test for the detection of sulfur compoun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine
Pyridine is a basic (chemistry), basic heterocyclic compound, heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties The molecular electric dipole moment is 2.2 debyes. Pyridine is diamagnetism, diamagnetic and has a Magnetic susceptibility, diamagnetic susceptibility of −48.7 × 10−6 cm3·mol−1. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy, treatment and antibiotic prophylaxis, prevention of such infections. They may either bactericide, kill or bacteriostatic agent, inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the common cold or influenza; drugs which inhibit viruses are termed antiviral drugs or antivirals rather than antibiotics. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek language, Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against microbes, but in the usual medical usage, antibiotics (such as penicillin) are those produced naturally (by one microorgani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfamethoxazole
Sulfamethoxazole (SMZ or SMX) is an antibiotic. It is used for bacterial infections such as urinary tract infections, bronchitis, and prostatitis and is effective against both gram negative and positive bacteria such as ''Listeria monocytogenes'' and '' E. coli''. Common side effects include nausea, vomiting, loss of appetite, and skin rashes. It is a sulfonamide and bacteriostatic. It resembles a component of folic acid. It prevents folic acid synthesis in the bacteria that must synthesize their own folic acid. Mammalian cells, and some bacteria, do not synthesize but require preformed folic acid (vitamin B9); they are therefore insensitive to sulfamethoxazole. It was introduced to the United States in 1961. It is now mostly used in combination with trimethoprim (abbreviated SMX-TMP). The SMX-TMP combination is on the WHO Model List of Essential medicines as a first-choice treatment for urinary tract infections. Other names include: sulfamethalazole, sulfisomezole,PubChem" ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sulfanilamide
Sulfanilamide (also spelled sulphanilamide) is a sulfonamide antibacterial drug. Chemically, it is an organic compound consisting of an aniline derivatized with a sulfonamide group. Powdered sulfanilamide was used by the Allies in World War II to reduce infection rates and contributed to a dramatic reduction in mortality rates compared to previous wars. Sulfanilamide is rarely if ever used systemically due to toxicity and because more effective sulfonamides are available for this purpose. Modern antibiotics have supplanted sulfanilamide on the battlefield; however, sulfanilamide remains in use today in the form of topical preparations, primarily for treatment of vaginal yeast infections mainly vulvovaginitis which is caused by ''Candida albicans''. The term "sulfanilamides" is also sometimes used to describe a family of molecules containing these functional groups. Examples include: * Furosemide, a loop diuretic * Sulfadiazine, an antibiotic * Sulfamethoxazole, an antibioti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Artificial Sweeteners
A sugar substitute is a food additive that provides a sweetness like that of sugar while containing significantly less food energy than sugar-based sweeteners, making it a zero-calorie () or low-calorie sweetener. Artificial sweeteners may be derived through manufacturing of plant extracts or processed by chemical synthesis. Sugar substitute products are commercially available in various forms, such as small pills, powders, and packets. In North America, common sugar substitutes include aspartame, monk fruit extract, saccharin, sucralose, and stevia; cyclamate is also used outside the United States. These sweeteners are a fundamental ingredient in diet drinks to sweeten them without adding calories. Additionally, sugar alcohols such as erythritol, xylitol, and sorbitol are derived from sugars. Approved artificial sweeteners do not cause cancer. Reviews and dietetic professionals have concluded that moderate use of non-nutritive sweeteners as a safe replacement for sugars ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saccharin
Saccharin (''aka'' saccharine, Sodium sacchari) is an artificial sweetener with effectively no nutritional value. It is about 550 times as sweet as sucrose but has a bitter or metallic aftertaste, especially at high concentrations. Saccharin is used to sweeten products such as drinks, candies, cookies, and especially for masking bitter taste of some medicines. Etymology Saccharin derives its name from the word "saccharine", meaning "sugary". The word saccharine is used figuratively, often in a derogative sense, to describe something "unpleasantly over-polite" or "overly sweet". Both words are derived from the Greek word (''sakkharon'') meaning "gravel". Similarly, saccharose is an obsolete name for sucrose (table sugar). Properties Saccharin is heat-stable. It does not react chemically with other food ingredients; as such, it stores well. Blends of saccharin with other sweeteners are often used to compensate for each sweetener's weaknesses and faults. A 10:1 cyclamate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
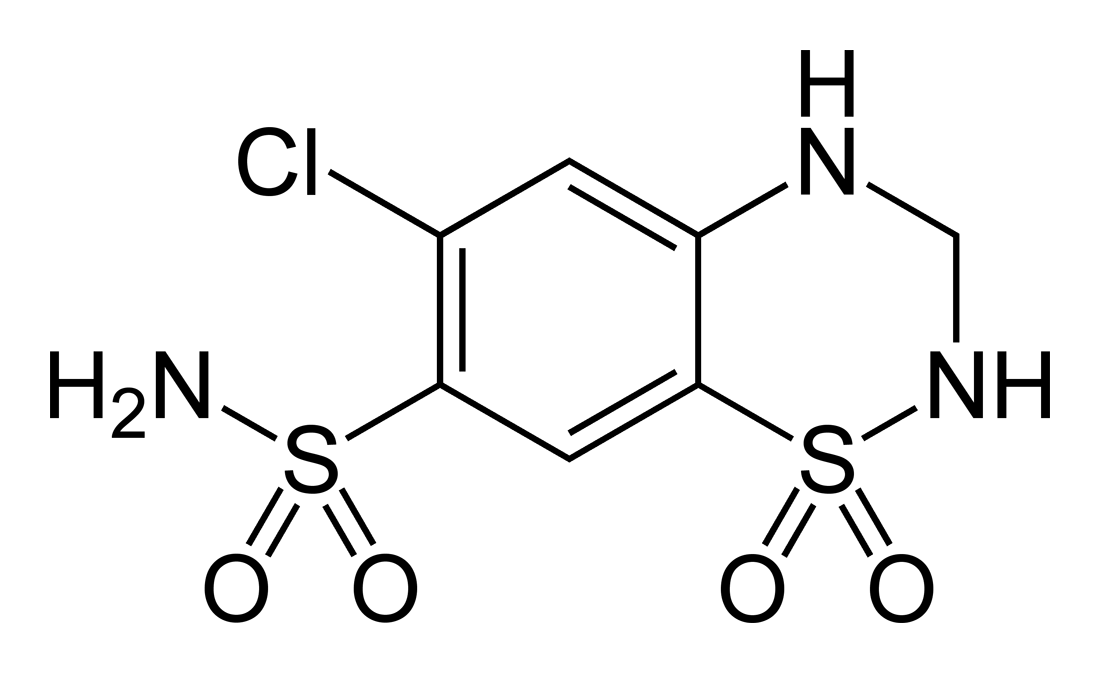
Sulthiame
Sultiame (or sulthiame) is a sulfonamide and inhibitor of the enzyme carbonic anhydrase. It is used as an anticonvulsant. History Sultiame was first synthesised in the laboratories of Bayer AG in the mid 1950s and eventually launched as Ospolot in Europe and other markets the early 1960s. It never became a registered drug in the United States. The brand was transferred to Desitin GmbH in 1993 and is sold in several European countries, in Israel, Japan, and Australia. Sultiame became established as a second-line drug for treatment of partial epilepsy in the 1960s and 1970s and was often used in combination with the established anticonvulsant phenytoin. Temporal lobe seizures appeared particularly responsive to sultiame. Doubts subsequently arose as to whether sultiame has intrinsic anticonvulsant properties. After discovering sultiame's ability to raise the blood levels of phenytoin, it was assumed that sultiame would only act in combination with phenytoin. This finding, togethe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ampiroxicam
Ampiroxicam ( INN) is a non-steroidal anti-inflammatory drug (NSAID). It is a prodrug of piroxicam Piroxicam is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class used to relieve the symptoms of painful inflammatory conditions like arthritis. Piroxicam works by preventing the production of endogenous prostaglandins which are i .... References Nonsteroidal anti-inflammatory drugs Prodrugs 2-Pyridyl compounds Benzothiazines Ethers {{analgesic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |