|
Sulfoxylate
Sulfoxylic acid (H2SO2) (also known as hyposulfurous acid or sulfur dihydroxide) is an unstable oxoacid of sulfur in an intermediate oxidation state between hydrogen sulfide and dithionous acid. It consists of two hydroxy groups attached to a sulfur atom. Sulfoxylic acid contains sulfur in an oxidation state of +2. Sulfur monoxide (SO) can be considered as a theoretical anhydride for sulfoxylic acid, but it is not actually known to react with water. The complementary base is the sulfoxylate anion which is much more stable. In between these states is the ion, also somewhat stable. Sulfoxylate ions can be made by decomposing thiourea dioxide in an alkaline solution. To do this, thiourea dioxide first forms an amidine-sulfinic acid tautomer, H2NC(=NH)SO2H, which then breaks apart. Sulfoxylate reacts with formaldehyde to yield a hydroxymethanesulfinate called rongalite: : + H2CO → , which is an important chemical for dyeing. Formation Sulfoxylic acid has been detected in the gas ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rongalite
Rongalite is a chemical compound with the molecular formula Na+HOCH2SO2−. This salt (chemistry), salt has many additional names, including Rongalit, sodium hydroxymethylsulfinate, sodium formaldehyde sulfoxylate, and Bruggolite. It is listed in the European Cosmetics Directive as sodium oxymethylene sulfoxylate (International Nomenclature of Cosmetic Ingredients, INCI). It is water-soluble and generally sold as the dihydrate. The compound and its derivative (chemistry), derivatives are widely used in the dye industry.D. Schubart "Sulfinic Acids and Derivatives" in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2012, Wiley-VCH, Weinheim. The structure of this salt has been confirmed by X-ray crystallography. Synthesis and reactions Although available commercially, the salt can be prepared from sodium dithionite and formaldehyde: :Na2S2O4 + 2 CH2O + H2O → HO-CH2-SO3Na + HO-CH2-SO2Na This reaction proceeds quantitatively, such that dithionite can be determined by ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Circumstellar Disk
A Circumstellar disc (or circumstellar disk) is a torus, pancake or ring-shaped accretion disk of matter composed of gas, dust, planetesimals, asteroids, or collision fragments in orbit around a star. Around the youngest stars, they are the reservoirs of material out of which planets may form. Around mature stars, they indicate that planetesimal formation has taken place, and around white dwarfs, they indicate that planetary material survived the whole of stellar evolution. Such a disc can manifest itself in various ways. Young star According to the widely accepted model of star formation, sometimes referred to as the nebular hypothesis, a young star (protostar) is formed by the gravitational collapse of a pocket of matter within a giant molecular cloud. The infalling material possesses some amount of angular momentum, which results in the formation of a gaseous protoplanetary disc around the young, rotating star. The former is a rotating circumstellar disc of dense gas and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
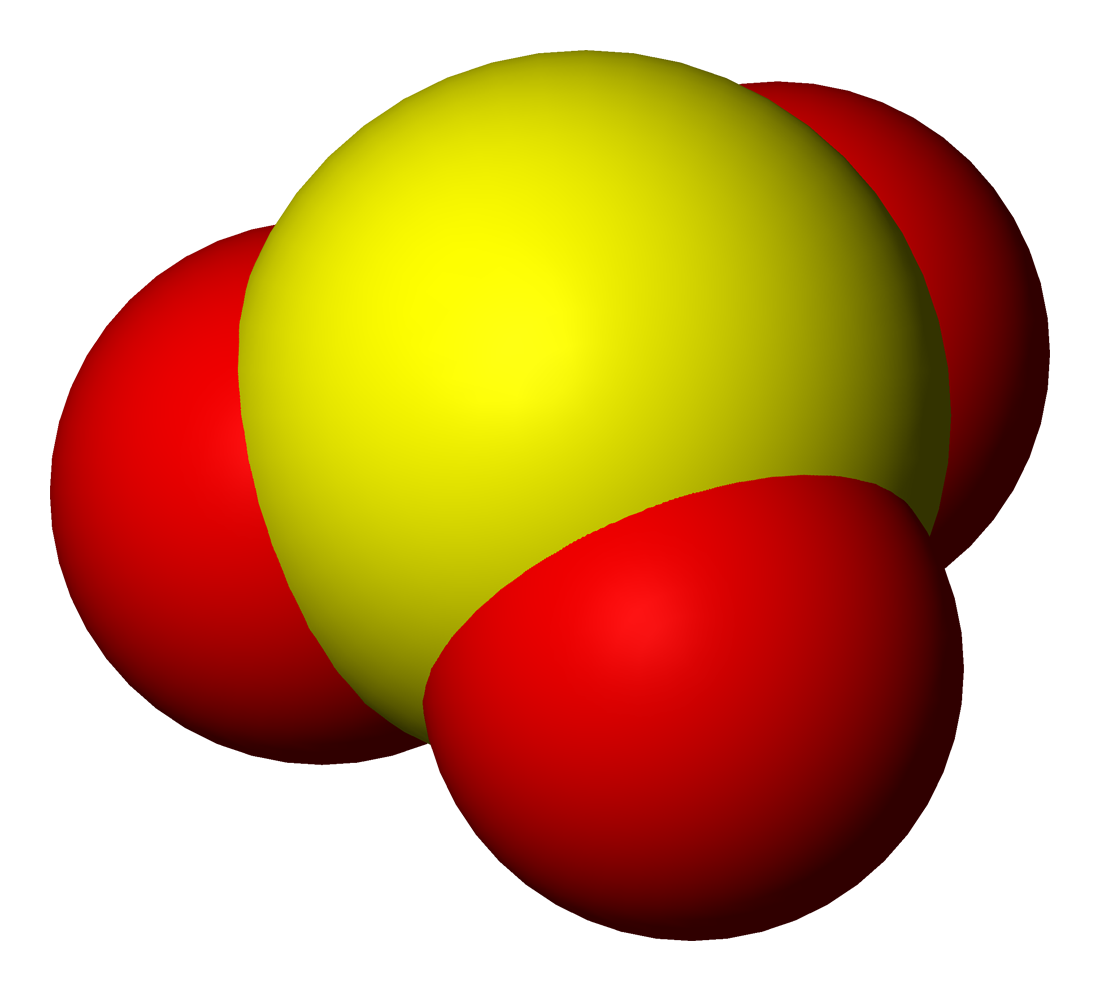
Sulfite
Sulfites or sulphites are compounds that contain the sulfite ion (systematic name: sulfate(IV) ion), . The sulfite ion is the conjugate base of bisulfite. Although its acid (sulfurous acid) is elusive, its salts are widely used. Sulfites are substances that naturally occur in some foods and the human body. They are also used as regulated food additives. When in food or drink, sulfites are often lumped together with sulfur dioxide.SeREGULATION (EU) No 1169/2011 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL/ref> Structure The structure of the sulfite anion can be described with three equivalent resonance structures. In each resonance structure, the sulfur atom is double-bonded to one oxygen atom with a formal charge of zero (neutral), and sulfur is singly bonded to the other two oxygen atoms, which each carry a formal charge of −1, together accounting for the −2 charge on the anion. There is also a non-bonded lone pair on the sulfur, so the structure predicted by VSEPR ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
X-ray Absorption Near Edge Structure
X-ray absorption near edge structure (XANES), also known as near edge X-ray absorption fine structure (NEXAFS), is a type of absorption spectroscopy that indicates the features in the X-ray absorption spectra ( XAS) of condensed matter due to the photoabsorption cross section for electronic transitions from an atomic core level to final states in the energy region of 50–100 eV above the selected atomic core level ionization energy, where the wavelength of the photoelectron is larger than the interatomic distance between the absorbing atom and its first neighbour atoms. Terminology Both XANES and NEXAFS are acceptable terms for the same technique. XANES name was invented in 1980 by Antonio Bianconi to indicate strong absorption peaks in X-ray absorption spectra in condensed matter due to multiple scattering resonances above the ionization energy. The name NEXAFS was introduced in 1983 by Jo Stohr and is synonymous with XANES, but is generally used when applied to surface and molec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dihydrogen Persulfoxide
Hydrogen is a chemical element; it has symbol H and atomic number 1. It is the lightest and most abundant chemical element in the universe, constituting about 75% of all normal matter. Under standard conditions, hydrogen is a gas of diatomic molecules with the formula , called dihydrogen, or sometimes hydrogen gas, molecular hydrogen, or simply hydrogen. Dihydrogen is colorless, odorless, non-toxic, and highly combustible. Stars, including the Sun, mainly consist of hydrogen in a plasma state, while on Earth, hydrogen is found as the gas (dihydrogen) and in molecular forms, such as in water and organic compounds. The most common isotope of hydrogen (H) consists of one proton, one electron, and no neutrons. Hydrogen gas was first produced artificially in the 17th century by the reaction of acids with metals. Henry Cavendish, in 1766–1781, identified hydrogen gas as a distinct substance and discovered its property of producing water when burned; hence its name means 'water-f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |