|
State Of Health
State of health (SoH) is a figure of merit of the condition of a battery (or a cell, or a battery pack), compared to its ideal conditions. The unit of SoH is percent (100% = the battery's conditions match the battery's specifications). For example, when the capacity of a new battery is same as the nominal capacity as per the battery specification, it is said to be in optimal health (SoH = 100%). As the battery is further utilized in a device, its health as in its capacity and other useful parameters deteriorate till it reaches the end of life (SoH = ~70-80%). Consequently, such batteries are replaced from regular usage pertaining to their unstable and unreliable performance. Typically, a battery's SoH will be 100% at the time of manufacture and will decrease over time and use. However, a battery's performance at the time of manufacture may not meet its specifications, in which case its initial SoH will be less than 100%. In the domain of electric vehicles, the biggest factors tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Figure Of Merit
A figure of merit (FOM) is a performance metric that characterizes the performance of a device, system, or method, relative to its alternatives. Examples *Absolute alcohol content per currency unit in an alcoholic beverage *accurizing, Accuracy of a rifle *Audio amplifier figures of merit such as gain or efficiency *Battery life of a laptop computer New York Times, June 25, 2009 *Calories per serving *Clock rate of a CPU is often given as a figure of merit, but is of limited use in comparing between different architectures. FLOPS may be a better figure, though these too are not completely representative of the performance of a CPU. *Contrast ratio of an LCD *Frequency response of a Loudspeaker, speaker *Fill factor (solar cell), Fill factor of a solar cell *Image resolutio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Battery Charger
A battery charger, recharger, or simply charger, is a device that stores energy in an electric battery by running current through it. The charging protocol—how much voltage and current, for how long and what to do when charging is complete—depends on the size and type of the battery being charged. Some battery types have high tolerance for overcharging after the battery has been fully charged and can be recharged by connection to a constant voltage source or a constant current source, depending on battery type. Simple chargers of this type must be manually disconnected at the end of the charge cycle. Other battery types use a timer to cut off when charging should be complete. Other battery types cannot withstand over-charging, becoming damaged (reduced capacity, reduced lifetime), over heating or even exploding. The charger may have temperature or voltage sensing circuits and a microprocessor controller to safely adjust the charging current and voltage, determine the stat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rechargeable Batteries
A rechargeable battery, storage battery, or secondary cell (formally a type of energy accumulator), is a type of electrical battery which can be charged, discharged into a load, and recharged many times, as opposed to a disposable or primary battery, which is supplied fully charged and discarded after use. It is composed of one or more electrochemical cells. The term "accumulator" is used as it accumulates and stores energy through a reversible electrochemical reaction. Rechargeable batteries are produced in many different shapes and sizes, ranging from button cells to megawatt systems connected to stabilize an electrical distribution network. Several different combinations of electrode materials and electrolytes are used, including lead–acid, zinc–air, nickel–cadmium (NiCd), nickel–metal hydride (NiMH), lithium-ion (Li-ion), lithium iron phosphate (LiFePO4), and lithium-ion polymer (Li-ion polymer). Rechargeable batteries typically initially cost more th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Battery Charging
A battery charger, recharger, or simply charger, is a device that energy storage, stores energy in an electric battery by running electric current, current through it. The charging protocol—how much voltage and current, for how long and what to do when charging is complete—depends on the size and type of the battery being charged. Some battery types have high tolerance for overcharging after the battery has been fully charged and can be recharged by connection to a constant voltage source or a constant current source, depending on battery type. Simple chargers of this type must be manually disconnected at the end of the charge cycle. Other battery types use a timer to cut off when charging should be complete. Other battery types cannot withstand over-charging, becoming damaged (reduced capacity, reduced lifetime), over heating or even exploding. The charger may have temperature or voltage sensing circuits and a microprocessor controller to safely adjust the charging current ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
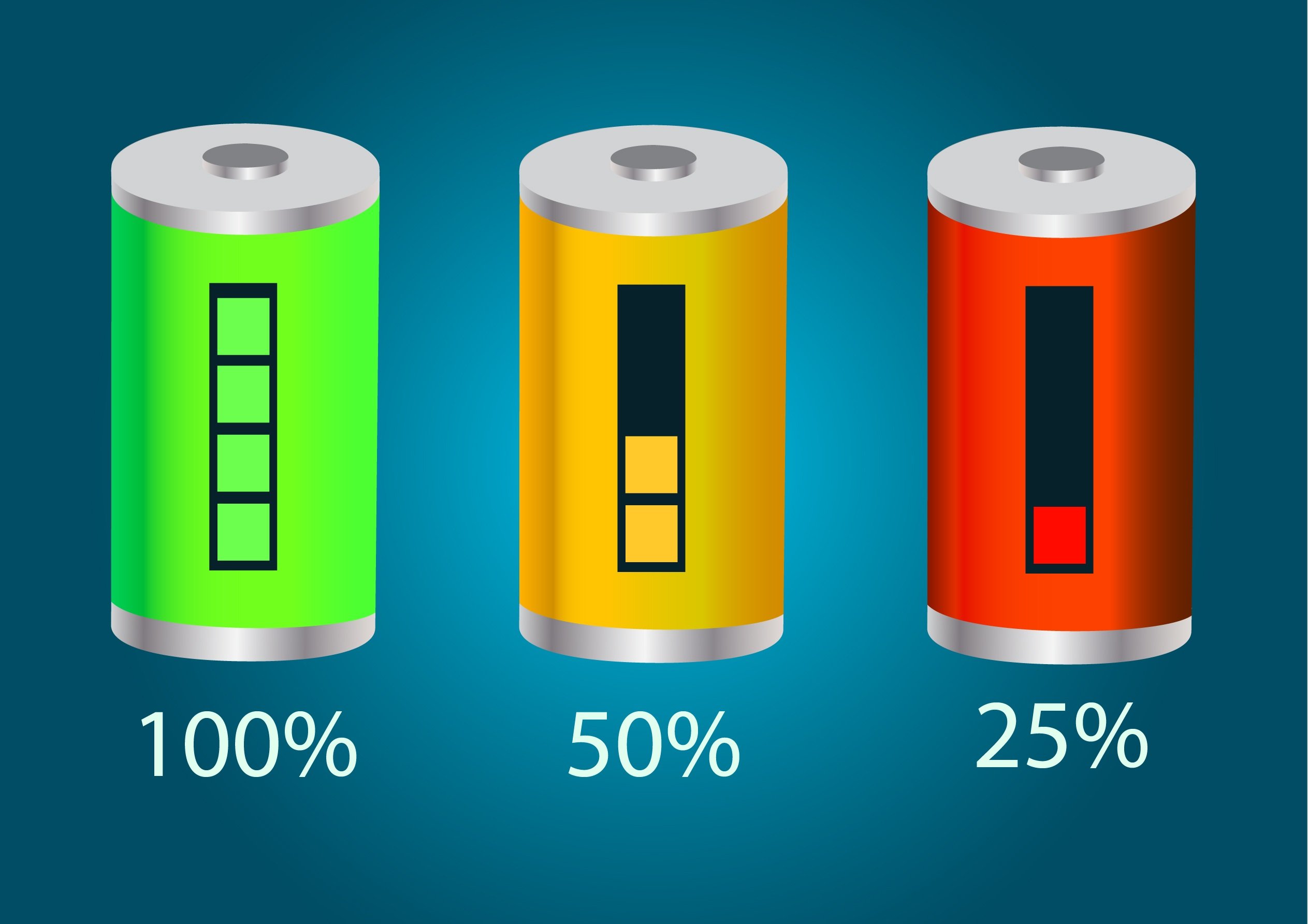
State Of Charge
State of charge (SOC) quantifies the remaining capacity available in a battery at a given time and in relation to a given state of ageing. It is usually expressed as percentage (0% = empty; 100% = full). An alternative form of the same measure is the depth of discharge (DOD), calculated as 1 − SOC (100% = empty; 0% = full). It refers to the amount of charge that may be used up if the cell is fully discharged. State of charge is normally used when discussing the present state of a battery in use, while depth of discharge is most often used to discuss a constant variation of state of charge during repeated cycles. In electric vehicles In a battery electric vehicle (BEV), the state of charge indicates the remaining energy in the battery pack. It is the equivalent of a fuel gauge. The state of charge can help to reduce electrical car owners' anxiety when they are waiting in the line or stay at home since it will reflect the progress of charging and let owners know when it wil ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recovery Effect
The recovery effect is a phenomenon observed in battery usage where the available energy is less than the difference between energy charged and energy consumed. Intuitively, this is because the energy has been consumed from the edge of the battery and the charge has not yet diffused evenly around the battery. When power is extracted continuously voltage decreases in a smooth curve, but the recovery effect can result in the voltage partially increasing if the current is interrupted. The KiBaM battery model describes the recovery effect for lead-acid batteries and is also a good approximation to the observed effects in Li-ion batteries. In some batteries, the gains from the recovery life can extend battery life by up to 45% by alternating discharging and inactive periods rather than constantly discharging. The size of the recovery effect depends on the battery load, recovery time and depth of discharge. Even though the recovery effect phenomenon is prominent in the lead acid batter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Depth Of Discharge
Depth of discharge (DoD) is an important parameter appearing in the context of rechargeable battery operation. Two non-identical definitions can be found in commercial and scientific sources. The depth of discharge is defined as: # the maximum fraction of a battery's capacity (given in Ah) which is removed from the charged battery on a regular basis. "''Charged"'' does not necessarily refer to ''fully or 100'' ''% charged'', but rather to the state of charge (SoC), where the battery charger stops charging, which is achieved by different techniques. # the fraction of the battery's capacity which is ''currently'' removed from the battery with regard to its (fully) charged state. For fully charged batteries, the depth of discharge is connected to the state of charge by the simple formula \mathrm = 1-\mathrm. The depth of discharge then is the complement of state of charge: as one increases, the other decreases. This definition is mostly found in scientific sources. The depth of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Battery Monitoring
A battery management system (BMS) is any electronic system that manages a rechargeable battery (cell or battery pack) by facilitating the safe usage and a long life of the battery in practical scenarios while monitoring and estimating its various states (such as state of health and state of charge), calculating secondary data, reporting that data, controlling its environment, authenticating or balancing it. Protection circuit module (PCM) is a simpler alternative to BMS. A battery pack built together with a BMS with an external communication data bus is a smart battery pack. A smart battery pack must be charged by a smart battery charger. Functions Monitor A BMS may monitor the state of the battery as represented by various items, such as: * Voltage: total voltage, voltages of individual cells, or voltage of * Temperature: average temperature, coolant intake temperature, coolant output temperature, or temperatures of individual cells * Coolant flow: for liquid cooled bat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Battery Fade
Capacity loss or capacity fading is a phenomenon observed in rechargeable battery usage where the amount of charge a battery can deliver at the rated voltage decreases with use. In 2003 it was reported the typical range of capacity loss in lithium-ion batteries after 500 charging and discharging cycles varied from 12.4% to 24.1%, giving an average capacity loss per cycle range of 0.025–0.048% per cycle. Stress factors Capacity fading in Li-ion batteries occurs by a multitude of stress factors, including ambient temperature, discharge C-rate, and state of charge (SOC). Capacity loss is strongly temperature-dependent, the aging rates increase with decreasing temperature below 25 °C, while above 25 °C aging is accelerated with increasing temperature. Capacity loss is C-rate sensitive and higher C-rates lead to a faster capacity loss on a per cycle. Chemical mechanisms of degradation in a Li-ion battery dominate capacity loss at low C-rates, whereas, mechanical degrada ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Battery Balancer
Battery balancing and battery redistribution refer to techniques that improve the available capacity of a battery pack with multiple cells (usually in series) and increase each cell's longevity. A ''battery balancer'' or ''regulator'' is an electrical device in a battery pack that performs battery balancing. Circuitry that includes designs to balance cell charges during battery pack recharging may be either ''active'' or ''passive'' in its design, and is most often found in lithium-ion batteries, e.g., for laptop computers, electrical vehicles. etc. Rationale The individual cells in a battery pack naturally have somewhat different capacities, and so, over the course of charge and discharge cycles, may be at a different state of charge (SOC). Variations in capacity are due to manufacturing variances, assembly variances (e.g., cells from one production run mixed with others), cell aging, impurities, or environmental exposure (e.g., some cells may be subject to additional ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Battery (electricity)
An electric battery is a source of electric power consisting of one or more electrochemical cells with external connections for powering electrical devices. When a battery is supplying power, its positive terminal is the cathode and its negative terminal is the anode. The terminal marked negative is the source of electrons. When a battery is connected to an external electric load, those negatively charged electrons flow through the circuit and reach the positive terminal, thus causing a redox reaction by attracting positively charged ions, or cations. Thus, higher energy reactants are converted to lower energy products, and the free-energy difference is delivered to the external circuit as electrical energy. Historically the term "battery" specifically referred to a device composed of multiple cells; however, the usage has evolved to include devices composed of a single cell. Primary (single-use or "disposable") batteries are used once and discarded, as the electrode mat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Self-discharge
Self-discharge is a phenomenon in batteries. Self-discharge decreases the shelf life Shelf life is the length of time that a commodity may be stored without becoming unfit for use, consumption, or sale. In other words, it might refer to whether a commodity should no longer be on a pantry shelf (unfit for use), or no longer on a s ... of batteries and causes them to have less than a full charge when actually put to use. How fast self-discharge in a battery occurs is dependent on the type of battery, state of charge, charging current, ambient temperature and other factors. Primary batteries are not designed for recharging between manufacturing and use, and thus to be practical they must have much lower self-discharge rates than older types of secondary cells. Later, secondary cells with similar very low self-discharge rates were developed, like low-self-discharge nickel–metal hydride cells. Self-discharge is a chemical reaction, just as closed-circuit discharge is, and tends ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |