|
Squaraine
1,3-Cyclobutanedione is an organic compound with the formula . It is an isomer of 1,2-Cyclobutanedione, 1,2-cyclobutanedione. The compound would be of little interest except that its tautomer is a subunit in some commercial dyes. In solution, 1,3-cyclobutanedione exists in equilibrium with a less stable tautomer, called squaraine, 3-hydroxycyclobut-2-enone. Squaraine dyes are, formally at least, derivatives of squaraine. In such dyes, the ene-one tautomer predominates. The mixture of tautomers can be prepared by hydrolysis of 1-ethoxycyclobutene-3-one, which is prepared from the cycloaddition of ethoxyacetylene to ketene. Substituted derivatives A variety of substituted 1,3-Cyclobutanediones form upon spontaneous dimerization of disubstituted ketenes. 2,2,4,4-Tetramethylcyclobutanedione is thus formed by dehydrochlorination of isobutyryl chloride: : : Ketene itself dimerizes mainly to give the lactone called diketene as well as a small amount of 1,3-cyclobutanedione. Relate ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Squaraine Dye
Squaraine dyes are a class of organic dyes showing intense fluorescence, typically in the red and near infrared region (absorption maxima are found between 630 and 670 nm and their emission maxima are between 650 and 700 nm). They are characterized by their unique aromatic four membered ring system derived from squaric acid. Most squaraines are wikt:encumbered, encumbered by nucleophilic attack of the central four membered ring, which is highly electron deficient. This encumbrance can be attenuated by the formation of a rotaxane around the dye to protect it from nucleophiles. They are currently used as sensors for ions and have recently, with the advent of protected squaraine derivatives, been exploited in biomedical imaging. Synthesis Synthesis of squaraine dyes was reported at least in 1966. They are derived from squaric acid which undergoes an electrophilic aromatic substitution reaction with an aniline or another electron rich derivative to form a highly conjugated p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
1,2-Cyclobutanedione
1,2-Cyclobutanedione is an organic compound with the formula . It is one of two isomers of cyclobutanedione, the other being 1,3-cyclobutanedione. It is prone to polymerization. It is prepared by desilylation of 1,2-bis(trimethylsiloxy)cyclobutene. Related compounds *Moniliformin Moniliformin is the organic compound with the formula (M+ = K+ or Na+). Both the sodium and potassium salts are generally hydrated, e.g. . In terms of its structure, it is the alkali metal salt of the conjugate base of 3-hydroxy-1,2-cyclobutene ..., a naturally occurring derivative of 1,2-butanedione References {{DEFAULTSORT:Cyclobutanedione, 1,2- Cyclobutanes Enols Diketones ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Tautomer
In chemistry, tautomers () are structural isomers (constitutional isomers) of chemical compounds that readily interconvert. The chemical reaction interconverting the two is called tautomerization. This conversion commonly results from the relocation of a hydrogen atom within the compound. The phenomenon of tautomerization is called tautomerism, also called desmotropism. Tautomerism is for example relevant to the behavior of amino acids and nucleic acids, two of the fundamental building blocks of life. Care should be taken not to confuse tautomers with depictions of "contributing structures" in chemical resonance. Tautomers are distinct chemical species that can be distinguished by their differing atomic connectivities, molecular geometries, and physicochemical and spectroscopic properties, whereas resonance forms are merely alternative Lewis structure (valence bond theory) depictions of a single chemical species, whose true structure is a quantum superposition, essentially the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
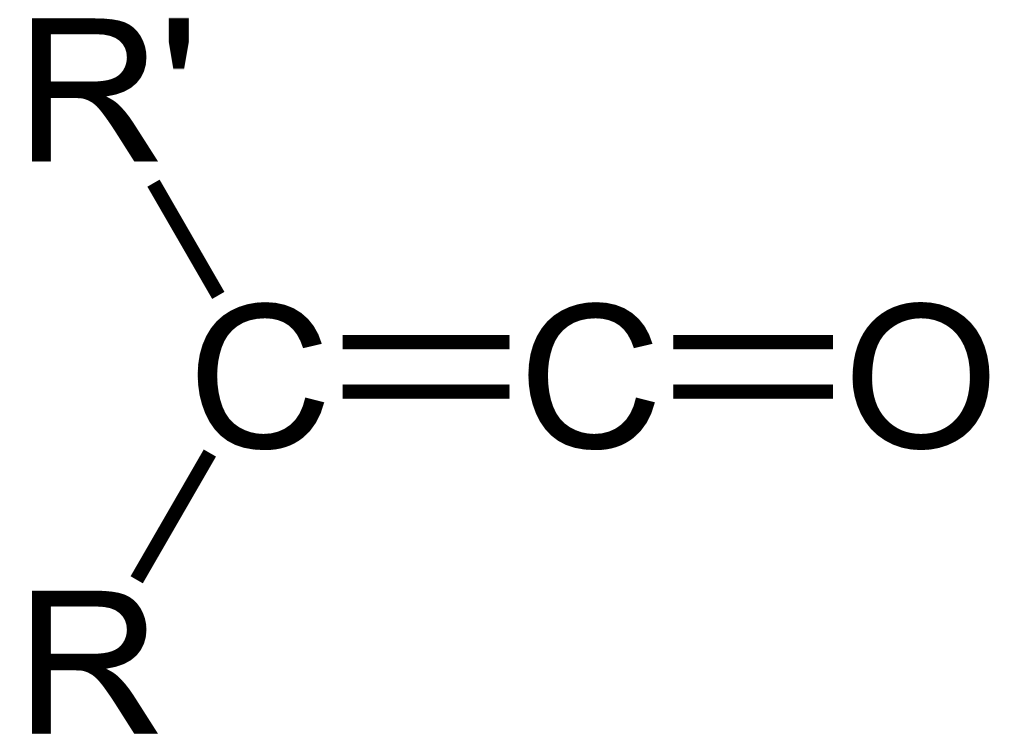
Ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The name may also refer to the specific compound ethenone , the simplest ketene. Although they are highly useful, most ketenes are chemical stability, unstable. When used as reagents in a chemical procedure, they are typically generated when needed, and consumed as soon as (or while) they are produced. History Ketenes were first studied as a class by Hermann Staudinger before 1905. Ketenes were systematically investigated by Hermann Staudinger in 1905 in the form of diphenylketene (conversion of \alpha-chlorodiphenyl acetyl chloride with zinc). Staudinger was inspired by the first examples of reactive organic intermediates and stable radicals discovered by Moses Gomberg in 1900 (compounds with triphenylmethyl group). Properties Ketenes are h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
2,2,4,4-Tetramethylcyclobutanedione
2,2,4,4-Tetramethylcyclobutanedione is the organic compound with the formula (CH3)4C4O2. The compound is a diketone of cyclobutane, bearing four methyl groups. It is a white solid that is used as a precursor to diverse industrial products. Synthesis and reactions 2,2,4,4-Tetramethylcyclobutanedione is the head-to-tail dimer of dimethylketene. It arises spontaneously when dimethylketene is produced by dehydrohalogenation of isobutyryl chloride with triethylamine. In the presence of aluminium trichloride, 2,2,4,4-tetramethylcyclobutanedione isomerizes to the lactone dimethylketene dimer (4-isopropylidene-3,3-dimethyl-2-oxetanone). Dimethylketene dimer is a precursor to various alkyl ketene dimers, which are used in papermaking. Hydrogenation Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Dehydrochlorination
In chemistry, dehydrohalogenation is an elimination reaction which removes a hydrogen halide from a substrate. The reaction is usually associated with the synthesis of alkenes, but it has wider applications. Dehydrohalogenation from alkyl halides Traditionally, alkyl halides are substrates for dehydrohalogenations. The alkyl halide must be able to form an alkene, thus halides having no C–H bond on an adjacent carbon are not suitable substrates. Aryl halides are also unsuitable. Upon treatment with strong base, chlorobenzene dehydrohalogenates to give phenol via a benzyne intermediate. Base-promoted reactions to alkenes When treated with a strong base many alkyl chlorides convert to corresponding alkene. It is also called a β-elimination reaction and is a type of elimination reaction. Some prototypes are shown below: :\begin \ce\ &\ce \\ \ce\ &\ce \\ \ce\ &\ce \end Here ethyl chloride reacts with potassium hydroxide, typically in a solvent such as ethanol, giving ethylene. Lik ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Isobutyryl Chloride
Isobutyryl chloride (2-methylpropanoyl chloride) is the organic compound with the formula . A colorless liquid, it the simplest branched-chain acyl chloride. It is prepared by chlorination of isobutyric acid. Reactions As an ordinary acid chloride, isobutyryl chloride is the subject of many reported transformations. Dehydrohalogenation of isobutyryl chloride with triethylamine gives 2,2,4,4-tetramethylcyclobutanedione. Treatment of isobutyryl chloride with hydrogen fluoride Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ... gives the acid fluoride. References Acyl chlorides Reagents for organic chemistry {{Organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Diketene
Diketene is an organic compound with the molecular formula , and which is sometimes written as . It is formed by dimerization of ketene, . Diketene is a member of the oxetane family. It is used as a reagent in organic chemistry. It is a colorless liquid. Production Diketene is produced on commercial scale by dimerization of ketene. Reactions Heating or irradiation with UV light regenerates the ketene monomer: : Alkylated ketenes also dimerize with ease and form substituted diketenes. Diketene readily hydrolyzes in water forming acetoacetic acid. Its half-life in water is approximately 45 min. a 25 °C at . Certain diketenes with two aliphatic chains, such as alkyl ketene dimers (AKDs), are used industrially to improve hydrophobicity in paper. At one time acetic anhydride was prepared by the reaction of ketene with acetic acid: : ΔH = −63 kJ/mol Acetoacetylation Diketene also reacts with alcohols and amines to the corresponding acetoacetic acid deri ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Moniliformin
Moniliformin is the organic compound with the formula (M+ = K+ or Na+). Both the sodium and potassium salts are generally hydrated, e.g. . In terms of its structure, it is the alkali metal salt of the conjugate base of 3-hydroxy-1,2-cyclobutenedione (the enolate of 1,2,3-cyclobutanetrione), a planar molecule related to squaric acid. It is an unusual mycotoxin, a feed contaminant that is lethal to fowl, especially ducklings. Moniliformin is formed in many cereals by a number of ''Fusarium'' species that include ''Fusarium moniliforme'', ''Fusarium avenaceum'', '' Fusarium subglutinans'', '' Fusarium proliferatum'', ''Fusarium fujikuroi'' and others. It is mainly cardiotoxic and causes ventricular hypertrophy. Biochemistry Moniliformin actually causes competitive inhibition of the activity of pyruvate dehydrogenase complex of respiratory reaction, which prevents pyruvic acid, product of glycolysis, to convert to acetyl-CoA. Ultrastructural examination of right ventricular wal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Cyclobutanes
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and is commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercial or biological significance, but more complex derivatives are important in biology and biotechnology. Structure The bond angles between carbon atoms are significantly strained and as such have lower bond energies than related linear or unstrained hydrocarbons, e.g. butane or cyclohexane. As such, cyclobutane is unstable above about 500 °C. The four carbon atoms in cyclobutane are not coplanar; instead, the ring typically adopts a folded or "puckered" conformation. This implies that the C-C-C angle is less than 90°. One of the carbon atoms makes a 25° angle with the plane formed by the other three carbons. In this way, some of the eclipsing interactions are reduced. The conformation is also known as a "butterfly". Equivalent p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |