|
Sperm Oil
Sperm oil (see also: Spermaceti) is a waxy liquid obtained from sperm whales. It is a clear, yellowish liquid with a very faint odor. Sperm oil has a different composition from common whale oil, obtained from rendered blubber. Although it is traditionally called an "oil", it is technically a liquid wax. It is composed of wax esters with a small proportion of triglycerides, an ester of an unsaturated fatty acid, and a branched-chain fatty alcohol. It is a natural antioxidant and heat transfer agent. In the late-18th and early-19th centuries, sperm oil was prized as an illuminant for its bright, odorless flame and as a lubricant for its low viscosity and stability. It was supplanted in the late 19th century by less expensive alternatives such as kerosene and petroleum-based lubricants. With the 1987 international ban on whaling, sperm oil is no longer legally sold. The oil from bottlenose whales was sometimes called "Arctic sperm oil." It was cheaper than but inferior to true ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sperm Oil Bottle And Can
Sperm (: sperm or sperms) is the male reproductive cell, or gamete, in anisogamous forms of sexual reproduction (forms in which there is a larger, female reproductive cell and a smaller, male one). Animals produce motile sperm with a tail known as a flagellum, which are known as spermatozoa, while some red algae and fungi produce non-motile sperm cells, known as spermatia. Flowering plants contain non-motile sperm inside pollen, while some more basal plants like ferns and some gymnosperms have motile sperm. Sperm cells form during the process known as spermatogenesis, which in amniotes (reptiles and mammals) takes place in the seminiferous tubules of the testicles. This process involves the production of several successive sperm cell precursors, starting with spermatogonia, which differentiate into spermatocytes. The spermatocytes then undergo meiosis, reducing their chromosome number by half, which produces spermatids. The spermatids then mature and, in animals, construct a t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bottlenose Whale
''Hyperoodon'' (or ''Hyperoödon'') is a genus of beaked whale, containing just two species: the Northern and Southern bottlenose whales. While not in the genus ''Hyperoodon'', Longman's beaked whales are alternatively called tropical bottlenose whales due to their physical features resembling those of bottlenose whales. They are considered to be molluscivorous, eating mainly squid A squid (: squid) is a mollusc with an elongated soft body, large eyes, eight cephalopod limb, arms, and two tentacles in the orders Myopsida, Oegopsida, and Bathyteuthida (though many other molluscs within the broader Neocoleoidea are also .... References Ziphiids {{whale-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cetyl Palmitate
Hexadecyl hexadecanoate, also known as cetyl palmitate, is the ester derived from hexadecanoic acid and 1-hexadecanol. This white waxy solid is the primary constituent of spermaceti, the once highly prized wax found in the skull of sperm whales.Wilhelm Riemenschneider and Hermann M. Bolt "Esters, Organic" Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. Cetyl palmitate is a component of some solid lipid nanoparticles. Stony corals, which build the coral reefs, contain large amounts of cetyl palmitate wax in their tissues, which may function in part as an antifeedant. Applications Cetyl palmitate is used in cosmetics as a thickener and emulsifier An emulsion is a mixture of two or more liquids that are normally immiscible (unmixable or unblendable) owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Althou .... References Fatty acid esters Palmitate esters ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Margarine
Margarine (, also , ) is a Spread (food), spread used for flavoring, baking, and cooking. It is most often used as a substitute for butter. Although originally made from animal fats, most margarine consumed today is made from vegetable oil. The spread was originally named ''oleomargarine'' from Latin for ''oleum'' (olive oil) and Greek language, Greek ''margarite'' ("pearl", indicating luster). The name was later shortened to ''margarine'', or sometimes ''oleo'' (particularly in the Deep South). Margarine consists of a water-in-fat emulsion, with tiny droplets of water dispersed uniformly throughout a fat phase (chemistry), phase in a stable solid form. While butter is made by concentrating the butterfat of milk through centrifugation, modern margarine is made through a more intensive processing of refined vegetable oil and water. Per US federal regulation, products must have a minimum fat content of 80% (with a maximum of 16% water) to be labeled as such in the United States, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
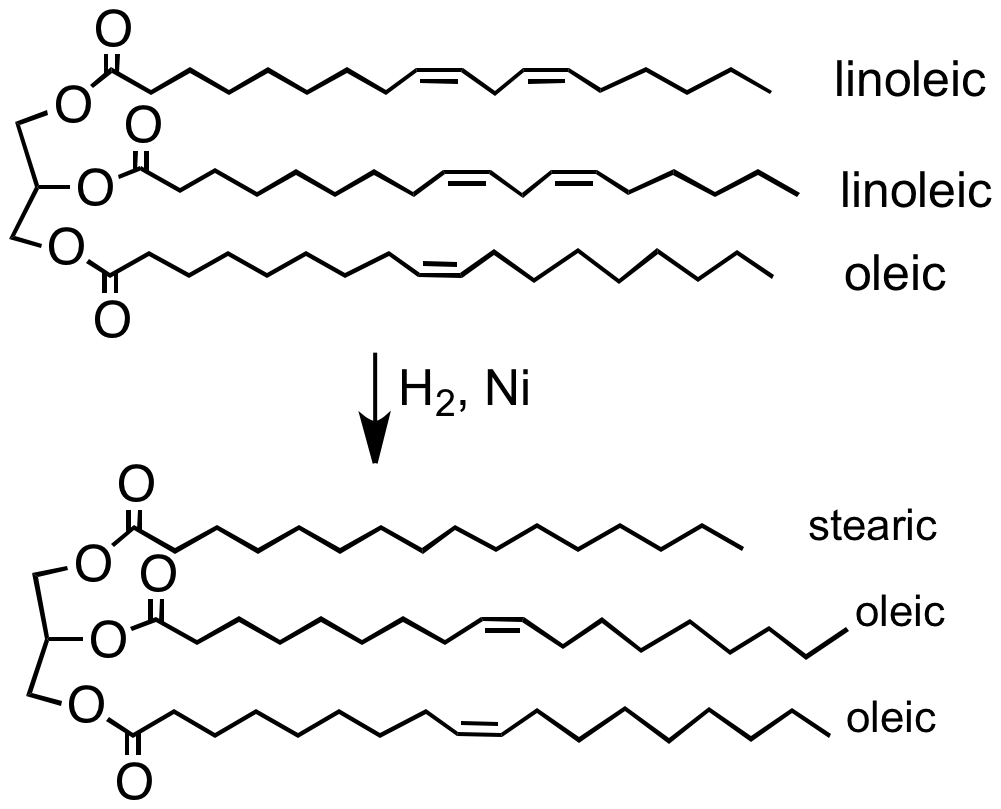
Hydrogenation
Hydrogenation is a chemical reaction between molecular hydrogen (H2) and another compound or element, usually in the presence of a catalyst such as nickel, palladium or platinum. The process is commonly employed to redox, reduce or Saturated and unsaturated compounds, saturate organic compounds. Hydrogenation typically constitutes the addition of pairs of hydrogen atoms to a molecule, often an alkene. Catalysts are required for the reaction to be usable; non-catalytic hydrogenation takes place only at very high temperatures. Hydrogenation reduces Double bond, double and Triple bond, triple bonds in hydrocarbons. Process Hydrogenation has three components, the Saturated and unsaturated compounds, unsaturated substrate, the hydrogen (or hydrogen source) and, invariably, a catalyst. The redox, reduction reaction is carried out at different temperatures and pressures depending upon the substrate and the activity of the catalyst. Related or competing reactions The same cataly ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coconut Oil
Coconut oil (or coconut fat) is an edible oil derived from the kernels, meat, and milk of the coconut palm fruit. Coconut oil is a white solid fat below around , and a clear thin liquid oil at higher temperatures. Unrefined varieties have a distinct coconut aroma. Coconut oil is used as a food oil, and in industrial applications for cosmetics and detergent production. The oil is rich in medium-chain fatty acids. Due to its high levels of saturated fat, numerous health authorities recommend limiting its consumption as a food. Coconut oil is widely used for cooking and baking due to its high smoke point and distinct flavor. Manufacturing Coconut oil can be extracted through a wet or dry process. More simply (but perhaps less effectively), oil can be produced by heating the meat via boiling water, the sun or a slow fire. Wet process thumb , Traditional () extraction directly from the milk in the latik">Philippines. The process also produces ''latik'' (curds), used as a G ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Viscosity Index
The viscosity index (VI) is an arbitrary, unit-less measure of a fluid's change in viscosity relative to temperature change. It is mostly used to characterize the viscosity-temperature behavior of lubricating oils. The lower the VI, the more the viscosity is affected by changes in temperature. The higher the VI, the more stable the viscosity remains over some temperature range. The VI was originally measured on a scale from 0 to 100; however, advancements in lubrication science have led to the development of oils with much higher VIs. The viscosity of a lubricant is closely related to its ability to reduce friction in solid body contacts. Generally, the least viscous lubricant which still forces the two moving surfaces apart to achieve " fluid bearing" conditions is desired. If the lubricant is too viscous, it will require a large amount of energy to move (as in honey); if it is too thin, the surfaces will come in contact and friction will increase. Relevance Many lubricant ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Iodine Number
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek , meaning 'violet'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compounds, it has also found favour as a non-toxic radiocontrast material. Because of the specificity of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Refractive Index
In optics, the refractive index (or refraction index) of an optical medium is the ratio of the apparent speed of light in the air or vacuum to the speed in the medium. The refractive index determines how much the path of light is bent, or refraction, refracted, when entering a material. This is described by Snell's law of refraction, , where and are the angle of incidence (optics), angle of incidence and angle of refraction, respectively, of a ray crossing the interface between two media with refractive indices and . The refractive indices also determine the amount of light that is reflectivity, reflected when reaching the interface, as well as the critical angle for total internal reflection, their intensity (Fresnel equations) and Brewster's angle. The refractive index, n, can be seen as the factor by which the speed and the wavelength of the radiation are reduced with respect to their vacuum values: the speed of light in a medium is , and similarly the wavelength in that me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Saponification Value
Saponification value or saponification number (SV or SN) represents the number of milligrams of potassium hydroxide (KOH) or sodium hydroxide (NaOH) required to saponify one gram of fat under the conditions specified. It is a measure of the average molecular weight (or chain length) of all the fatty acids present in the sample in form of triglycerides. The higher the saponification value, the lower the fatty acids average length, the lighter the mean molecular weight of triglycerides and vice versa. Practically, fats or oils with high saponification value (such as coconut and palm oil) are more suitable for soap making. Determination To determine saponification value, the sample is treated with an excess of alkali (usually an ethanolic solution of potassium hydroxide) for half an hour under reflux. The KOH is consumed by reaction with triglycerides, which consume three equivalents of base. Diglycerides consume two equivalents of KOH. Monoglycerides and free fatty acids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Flash Point
The flash point of a material is the "lowest liquid temperature at which, under certain standardized conditions, a liquid gives off vapours in a quantity such as to be capable of forming an ignitable vapour/air mixture". The flash point is sometimes confused with the autoignition temperature, the temperature that causes spontaneous ignition. The fire point is the lowest temperature at which the vapors keep burning after the ignition source is removed. It is higher than the flash point, because at the flash point vapor may not be produced fast enough to sustain combustion. Neither flash point nor fire point depends directly on the ignition source temperature, but ignition source temperature is far higher than either the flash or fire point, and can increase the temperature of fuel above the usual ambient temperature to facilitate ignition. Fuels The flash point is a descriptive characteristic that is used to distinguish between flammable fuels, such as petrol (also known as g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Gravity
Relative density, also called specific gravity, is a dimensionless quantity defined as the ratio of the density (mass of a unit volume) of a substance to the density of a given reference material. Specific gravity for solids and liquids is nearly always measured with respect to water at its densest (at ); for gases, the reference is air at room temperature (). The term "relative density" (abbreviated r.d. or RD) is preferred in SI, whereas the term "specific gravity" is gradually being abandoned. If a substance's relative density is less than 1 then it is less dense than the reference; if greater than 1 then it is denser than the reference. If the relative density is exactly 1 then the densities are equal; that is, equal volumes of the two substances have the same mass. If the reference material is water, then a substance with a relative density (or specific gravity) less than 1 will float in water. For example, an ice cube, with a relative density of about 0.91, will float. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |