|
Solvent Models
In computational chemistry, a solvent model is a computational method that accounts for the behavior of solvated condensed phases. Solvent models enable simulations and thermodynamic calculations applicable to reactions and processes which take place in solution. These include biological, chemical and environmental processes. Such calculations can lead to new predictions about the physical processes occurring by improved understanding. Solvent models have been extensively tested and reviewed in the scientific literature. The various models can generally be divided into two classes, explicit and implicit models, all of which have their own advantages and disadvantages. Implicit models are generally computationally efficient and can provide a reasonable description of the solvent behavior, but fail to account for the local fluctuations in solvent density around a solute molecule. The density fluctuation behavior is due to solvent ordering around a solute and is particularly prevalent w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Computational Chemistry
Computational chemistry is a branch of chemistry that uses computer simulations to assist in solving chemical problems. It uses methods of theoretical chemistry incorporated into computer programs to calculate the structures and properties of molecules, groups of molecules, and solids. The importance of this subject stems from the fact that, with the exception of some relatively recent findings related to the hydrogen molecular ion (dihydrogen cation), achieving an accurate quantum mechanical depiction of chemical systems analytically, or in a closed form, is not feasible. The complexity inherent in the many-body problem exacerbates the challenge of providing detailed descriptions of quantum mechanical systems. While computational results normally complement information obtained by chemical experiments, it can occasionally predict unobserved chemical phenomena. Overview Computational chemistry differs from theoretical chemistry, which involves a mathematical description of chem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monte Carlo Method
Monte Carlo methods, or Monte Carlo experiments, are a broad class of computational algorithms that rely on repeated random sampling to obtain numerical results. The underlying concept is to use randomness to solve problems that might be deterministic in principle. The name comes from the Monte Carlo Casino in Monaco, where the primary developer of the method, mathematician Stanisław Ulam, was inspired by his uncle's gambling habits. Monte Carlo methods are mainly used in three distinct problem classes: optimization, numerical integration, and generating draws from a probability distribution. They can also be used to model phenomena with significant uncertainty in inputs, such as calculating the risk of a nuclear power plant failure. Monte Carlo methods are often implemented using computer simulations, and they can provide approximate solutions to problems that are otherwise intractable or too complex to analyze mathematically. Monte Carlo methods are widely used in va ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solvents
A solvent (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for polar molecules, and the most common solvent used by living things; all the ions and proteins in a cell are dissolved in water within the cell. Major uses of solvents are in paints, paint removers, inks, and dry cleaning. Specific uses for organic solvents are in dry cleaning (e.g. tetrachloroethylene); as paint thinners (toluene, turpentine); as nail polish removers and solvents of glue (acetone, methyl acetate, ethyl acetate); in spot removers (hexane, petrol ether); in detergents ( citrus terpenes); and in perfumes (ethanol). Solvents find various applications in chemical, pharmaceutical, oil, and gas industries, including in chemical syntheses and purification processes Some petrochemical solvents are highly toxic and emit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
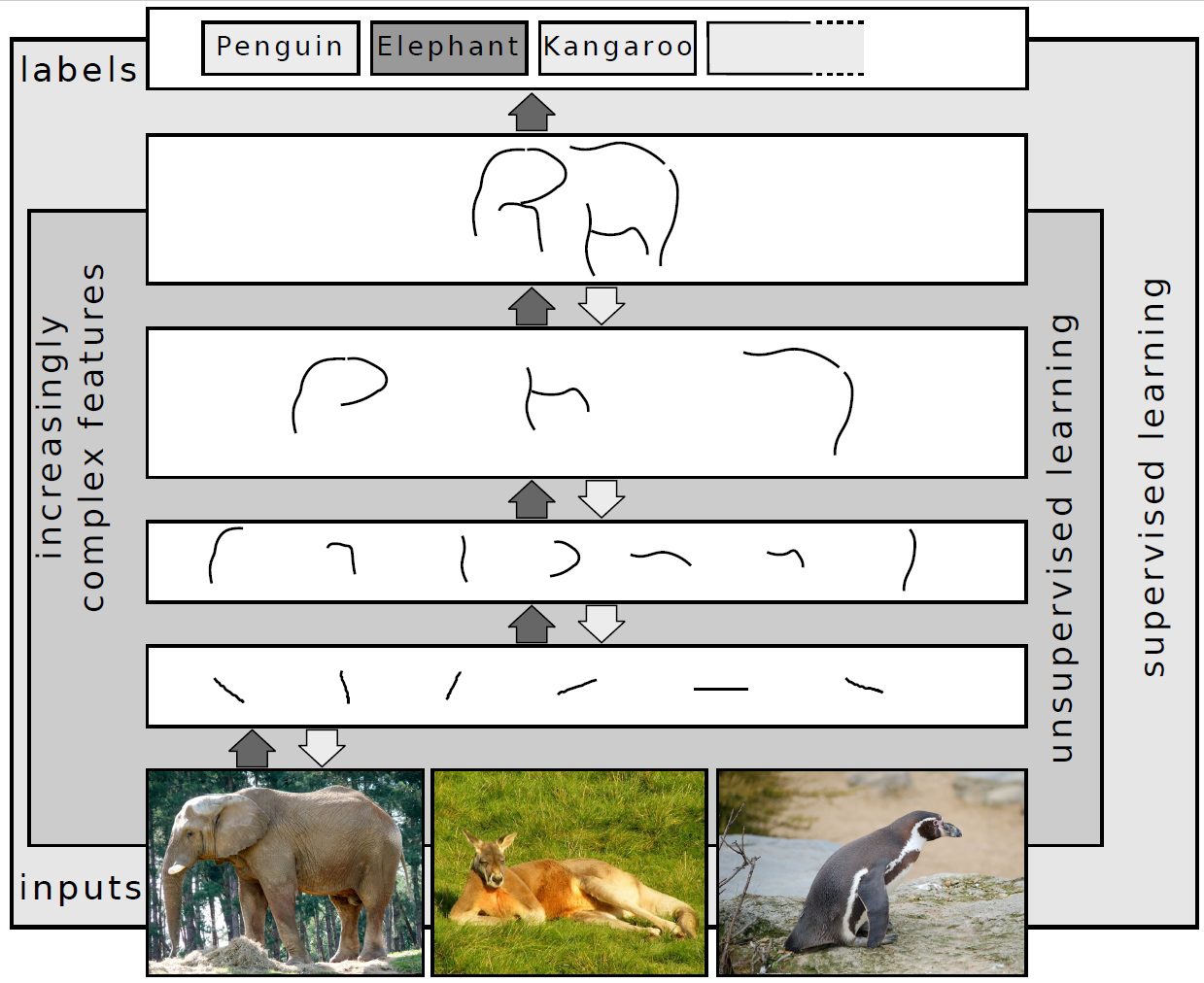
Deep Learning
Deep learning is a subset of machine learning that focuses on utilizing multilayered neural networks to perform tasks such as classification, regression, and representation learning. The field takes inspiration from biological neuroscience and is centered around stacking artificial neurons into layers and "training" them to process data. The adjective "deep" refers to the use of multiple layers (ranging from three to several hundred or thousands) in the network. Methods used can be either supervised, semi-supervised or unsupervised. Some common deep learning network architectures include fully connected networks, deep belief networks, recurrent neural networks, convolutional neural networks, generative adversarial networks, transformers, and neural radiance fields. These architectures have been applied to fields including computer vision, speech recognition, natural language processing, machine translation, bioinformatics, drug design, medical image analysis, c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
UNIFAC
In statistical thermodynamics, the UNIFAC method ( UNIQUAC Functional-group Activity Coefficients)Aage Fredenslund, Russell L. Jones and John M. Prausnitz, "Group-Contribution Estimation of Activity Coefficients in Nonideal Liquid Mixtures", ''AIChE Journal'', vol. 21 (1975), p. 1086 is a semi-empirical system for the prediction of non-electrolyte activity in non-ideal mixtures. UNIFAC uses the functional groups present on the molecules that make up the liquid mixture to calculate activity coefficients. By using interactions for each of the functional groups present on the molecules, as well as some binary interaction coefficients, the activity of each of the solutions can be calculated. This information can be used to obtain information on liquid equilibria, which is useful in many thermodynamic calculations, such as chemical reactor design, and distillation calculations. The UNIFAC model was first published in 1975 by Fredenslund, Jones and John Prausnitz, a group of chemical ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
COSMO-RS
COSMO-RS (short for "Conductor-like Screening Model for Real Solvents")"Conductor-like Screening Model for Real Solvents: A New Approach to the Quantitative Calculation of Solvation Phenomena", A. Klamt, J. Phys. Chem., 99, 2224-2235 (1995), DOI: 10.1021/j100007a062/ref> is a quantum chemistry based equilibrium thermodynamics method with the purpose of predicting chemical potentials μ in liquids. It processes the screening charge density σ on the surface of molecules to calculate the chemical potential μ of each species in solution. Perhaps in dilute solution a constant potential must be considered. As an initial step a quantum chemical COSMO calculation for all molecules is performed and the results (e.g. the screening charge density) are stored in a database. In a separate step COSMO-RS uses the stored COSMO results to calculate the chemical potential of the molecules in a liquid solvent or mixture. The resulting chemical potentials are the basis for other thermodynamic equili ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radial Distribution Function
In statistical mechanics, the radial distribution function, (or pair correlation function) g(r) in a system of particles (atoms, molecules, colloids, etc.), describes how density varies as a function of distance from a reference particle. If a given particle is taken to be at the origin ''O'', and if \rho = N/V is the average number density of particles, then the local time-averaged density at a distance r from ''O'' is \rho g(r). This simplified definition holds for a homogeneous and isotropic system. A more general case will be considered below. In simplest terms it is a measure of the probability of finding a particle at a distance of r away from a given reference particle, relative to that for an ideal gas. The general algorithm involves determining how many particles are within a distance of r and r+dr away from a particle. This general theme is depicted to the right, where the red particle is our reference particle, and the blue particles are those whose centers are with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
RISM Matlab
Rism is a hamlet in Dhofar Governorate, in southwestern Oman.National Geospatial-Intelligence Agency. GeoNames GeoNames (or GeoNames.org) is a user-editable geographical database available and accessible through various web services, under a Creative Commons attribution license. The project was founded in late 2005. The GeoNames dataset differs from, b ... database entry.search Accessed 12 May 2011. References Populated places in the Dhofar Governorate {{Oman-geo-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
QM/MM
The hybrid QM/MM (quantum mechanics/molecular mechanics) approach is a molecular simulation method that combines the strengths of ''ab initio'' QM calculations (accuracy) and MM (speed) approaches, thus allowing for the study of chemical processes in solution and in proteins. The QM/MM approach was introduced in the 1976 paper of Warshel and Levitt. They, along with Martin Karplus, won the 2013 Nobel Prize in Chemistry for "the development of multiscale models for complex chemical systems". Efficiency An important advantage of QM/MM methods is their efficiency. The cost of doing classical molecular mechanics (MM) simulations in the most straightforward case scales as ''O''(''N''2), where ''N'' is the number of atoms in the system. This is mainly due to electrostatic interactions term (every particle interacts with everything else). However, use of cutoff radius, periodic pair-list updates and more recently the variations of the particle mesh Ewald (PME) method has reduced th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lennard-Jones Potential
In computational chemistry, molecular physics, and physical chemistry, the Lennard-Jones potential (also termed the LJ potential or 12-6 potential; named for John Lennard-Jones) is an intermolecular pair potential. Out of all the intermolecular potentials, the Lennard-Jones potential is probably the one that has been the most extensively studied. It is considered an archetype model for simple yet realistic intermolecular interactions. The Lennard-Jones potential is often used as a building block in molecular models (a.k.a. force fields) for more complex substances. Many studies of the idealized "Lennard-Jones substance" use the potential to understand the physical nature of matter. Overview The Lennard-Jones potential is a simple model that still manages to describe the essential features of interactions between simple atoms and molecules: Two interacting particles repel each other at very close distance, attract each other at moderate distance, and eventually stop intera ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |