|
Serum Chloride
Chloride is an anion in the human body needed for metabolism (the process of turning food into energy). It also helps keep the body's acid-base balance. The amount of serum chloride is carefully controlled by the kidneys. Chloride ions have important physiological roles. For instance, in the central nervous system, the inhibitory action of glycine and some of the action of GABA relies on the entry of Cl− into specific neurons. Also, the chloride-bicarbonate exchanger biological transport protein relies on the chloride ion to increase the blood's capacity of carbon dioxide, in the form of the bicarbonate ion; this is the mechanism underpinning the chloride shift occurring as the blood passes through oxygen-consuming capillary beds. The normal blood reference range of chloride for adults in most labs is 96 to 106 milliequivalents (mEq) per liter. The normal range may vary slightly from lab to lab. Normal ranges are usually shown next to results in the lab report. A diagnos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pronunciation of the word "chloride" is . Chloride salts such as sodium chloride are often soluble in water.Green, John, and Sadru Damji. "Chapter 3." ''Chemistry''. Camberwell, Vic.: IBID, 2001. Print. It is an essential electrolyte located in all body fluids responsible for maintaining acid/base balance, transmitting nerve impulses and regulating liquid flow in and out of cells. Other examples of ionic chlorides include potassium chloride (), calcium chloride (), and ammonium chloride (). Examples of covalent chlorides include methyl chloride (), carbon tetrachloride (), sulfuryl chloride (), and monochloramine (). Electronic properties A chloride ion (diameter 167 pm) is much larger than a chlorine atom (diameter 99 pm ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dietary Reference Intake
The Dietary Reference Intake (DRI) is a system of nutrition recommendations from the National Academy of Medicine (NAM) of the National Academies (United States). It was introduced in 1997 in order to broaden the existing guidelines known as Recommended Dietary Allowances (RDAs, see below). The DRI values differ from those used in nutrition labeling on food and dietary supplement products in the U.S. and Canada, which uses Reference Daily Intakes (RDIs) and Daily Values (%DV) which were based on outdated RDAs from 1968 but were updated as of 2016. Parameters DRI provides several different types of reference values: * Estimated Average Requirements (EAR), are expected to satisfy the needs of 50% of the people in that age group based on a review of the scientific literature. * Recommended Dietary Allowances (RDA), the daily dietary intake level of a nutrient considered sufficient by the Food and Nutrition Board of the Institute of Medicine to meet the requirements of 97.5% of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloridometer
A chloridometer is a measuring instrument used to determine the concentration of chloride ions (Cl–) in a Solution (chemistry), solution. It uses a process known as coulometric titration or ''amperostatic coulometry'', the accepted electrochemistry reference method to determine the concentration of chloride in biological fluids, including blood serum, blood plasma, urine, Perspiration, sweat, and cerebrospinal fluid. The coulometry process generates silver ions, which react with the chloride to form silver chloride (AgCl). The first chloridometer was designed by a team led by Ernest Cotlove in 1958. Other methods to determine chloride concentration include photometric titration and isotope dilution mass spectrometry. Operation An amperostat delivers a constant Electric current, current of about 6—8 Ampere, mA to the generator electrodes for the titration of the solution, and a digital timer is started. A second pair of silver electrodes are used as a detector to measure t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Equivalent (chemistry)
An equivalent (symbol: officially equiv; unofficially but often Eq) is the amount of a substance that reacts with (or is ''equivalent'' to) an arbitrary amount (typically one mole) of another substance in a given chemical reaction. It is an archaic quantity that was used in chemistry and the biological sciences (see '). The mass of an equivalent is called its equivalent weight. Formula The formula from milligrams (mg) to milli-equivalent (mEq) and back is as follows: \begin \text \to \text &: \quad \text \times \frac \\ pt\text \to \text &: \quad \text \times \frac \end where is the valence and is the molecular weight. For elemental compounds: \text \to \text : \quad \frac \times \frac Common examples mEq to milligram Milligram to mEq Formal definition In a more formal definition, the ''equivalent'' is the amount of a substance needed to do one of the following: * react with or supply one mole of hydrogen ions () in an acid–base reaction * react with or supply o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood Values
Reference ranges (reference intervals) for blood tests are sets of values used by a health professional to interpret a set of medical test results from blood samples. Reference ranges for blood tests are studied within the field of clinical chemistry (also known as "clinical biochemistry", "chemical pathology" or "pure blood chemistry"), the area of pathology that is generally concerned with analysis of bodily fluids. Blood test results should always be interpreted using the reference range provided by the laboratory that performed the test. Interpretation A reference range is usually defined as the set of values 95 percent of the normal population falls within (that is, 95% prediction interval). It is determined by collecting data from vast numbers of laboratory tests. Plasma or whole blood In this article, all values (except the ones listed below) denote blood plasma concentration, which is approximately 60–100% larger than the actual blood concentration if the amount inside ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
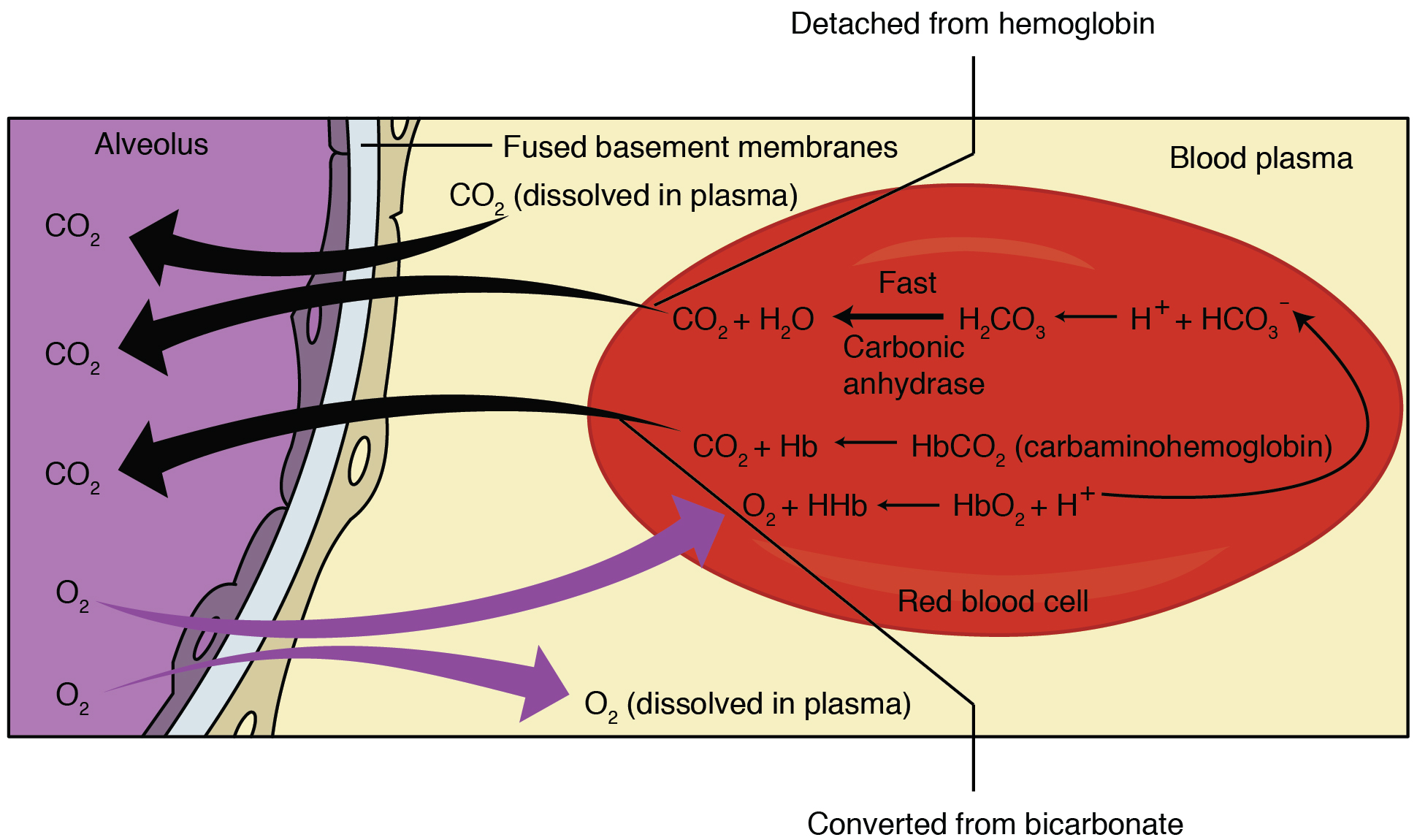
Chloride Shift
Chloride shift (also known as the Hamburger phenomenon or lineas phenomenon, named after Hartog Jakob Hamburger) is a process which occurs in a cardiovascular system and refers to the exchange of bicarbonate (HCO3−) and chloride (Cl−) across the membrane of red blood cells (RBCs). Mechanism Carbon dioxide (CO2) is produced in tissues as a byproduct of normal aerobic metabolism. It dissolves in the solution of blood plasma and into red blood cells (RBC), where carbonic anhydrase catalyzes its hydration to carbonic acid (H2CO3). Carbonic acid then spontaneously dissociates to form bicarbonate Ions (HCO3−) and a hydrogen ion (H+). In response to the decrease in intracellular pCO2, more CO2 passively diffuses into the cell. Cell membranes are generally impermeable to charged ions (i.e. H+, HCO3− ) but RBCs are able to exchange bicarbonate for chloride using the anion exchanger protein Band 3. Thus, the rise in intracellular bicarbonate leads to bicarbonate export and chlor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicarbonate
In inorganic chemistry, bicarbonate (IUPAC-recommended nomenclature: hydrogencarbonate) is an intermediate form in the deprotonation of carbonic acid. It is a polyatomic anion with the chemical formula . Bicarbonate serves a crucial biochemical role in the physiological pH buffering system. The term "bicarbonate" was coined in 1814 by the English chemist William Hyde Wollaston. The name lives on as a trivial name. Chemical properties The bicarbonate ion (hydrogencarbonate ion) is an anion with the empirical formula and a molecular mass of 61.01 daltons; it consists of one central carbon atom surrounded by three oxygen atoms in a trigonal planar arrangement, with a hydrogen atom attached to one of the oxygens. It is isoelectronic with nitric acid (). The bicarbonate ion carries a negative one formal charge and is an amphiprotic species which has both acidic and basic properties. It is both the conjugate base of carbonic acid (); and the conjugate acid of , t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Blood
Blood is a body fluid in the circulatory system of humans and other vertebrates that delivers necessary substances such as nutrients and oxygen to the cells, and transports metabolic waste products away from those same cells. Blood is composed of blood cells suspended in blood plasma. Plasma, which constitutes 55% of blood fluid, is mostly water (92% by volume), and contains proteins, glucose, mineral ions, and hormones. The blood cells are mainly red blood cells (erythrocytes), white blood cells (leukocytes), and (in mammals) platelets (thrombocytes). The most abundant cells are red blood cells. These contain hemoglobin, which facilitates oxygen transport by reversibly binding to it, increasing its solubility. Jawed vertebrates have an adaptive immune system, based largely on white blood cells. White blood cells help to resist infections and parasites. Platelets are important in the clotting of blood. Blood is circulated around the body through blood vessels by the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convention. The net charge of an ion is not zero because its total number of electrons is unequal to its total number of protons. A cation is a positively charged ion with fewer electrons than protons (e.g. K+ ( potassium ion)) while an anion is a negatively charged ion with more electrons than protons (e.g. Cl− ( chloride ion) and OH− ( hydroxide ion)). Opposite electric charges are pulled towards one another by electrostatic force, so cations and anions attract each other and readily form ionic compounds. Ions consisting of only a single atom are termed ''monatomic ions'', ''atomic ions'' or ''simple ions'', while ions consisting of two or more atoms are termed polyatomic ions or ''molecular ions''. If only a + or − is present, it indi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metabolic reactions, DNA replication, Cell signaling, responding to stimuli, providing Cytoskeleton, structure to cells and Fibrous protein, organisms, and Intracellular transport, transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the Nucleic acid sequence, nucleotide sequence of their genes, and which usually results in protein folding into a specific Protein structure, 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than 20–30 residues, are rarely considered to be proteins and are commonly called pep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloride-bicarbonate Exchanger
Band 3 anion transport protein, also known as anion exchanger 1 (AE1) or band 3 or solute carrier family 4 member 1 (SLC4A1), is a protein that is encoded by the gene in humans. Band 3 anion transport protein is a phylogenetically-preserved transport protein responsible for mediating the exchange of chloride (Cl−) with bicarbonate (HCO3−) across plasma membranes. Functionally similar members of the AE clade are AE2 and AE3. Function Band 3 is present in the basolateral face of the α-intercalated cells of the collecting ducts of the nephron, which are the main acid-secreting cells of the kidney. They generate hydrogen ions and bicarbonate ions from carbon dioxide and water – a reaction catalysed by carbonic anhydrase. The hydrogen ions are pumped into the collecting duct tubule by vacuolar H+ ATPase, the apical proton pump, which thus excretes acid into the urine. kAE1, the kidney isoform of AE1, exchanges bicarbonate for chloride on the basolateral surface, essen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |