|
Ridinilazole
Ridinilazole (previously known as SMT19969) is an investigational small molecule antibiotic under evaluation for oral administration for the treatment of Clostridioides difficile infection, ''Clostridioides difficile'' infection (CDI). In vitro, it demonstrates bactericidal activity against ''C. difficile'' and suppresses bacterial toxin production; the mechanism of action is thought to involve inhibition of cell division. It possesses desirable properties for the treatment of CDI, namely that it is a narrow-spectrum antibiotic which exhibits activity against ''C. difficile'' while having little impact on other normal intestinal flora and that it is only minimally absorbed systemically after oral administration. When Ridinilazole was originally developed, there were only three antibiotics in use for treating CDI: vancomycin, fidaxomicin, and metronidazole. The recurrence rate of CDI is high, which has spurred research into other treatment options with the aimed reduce the rate of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Clostridioides Difficile Infection
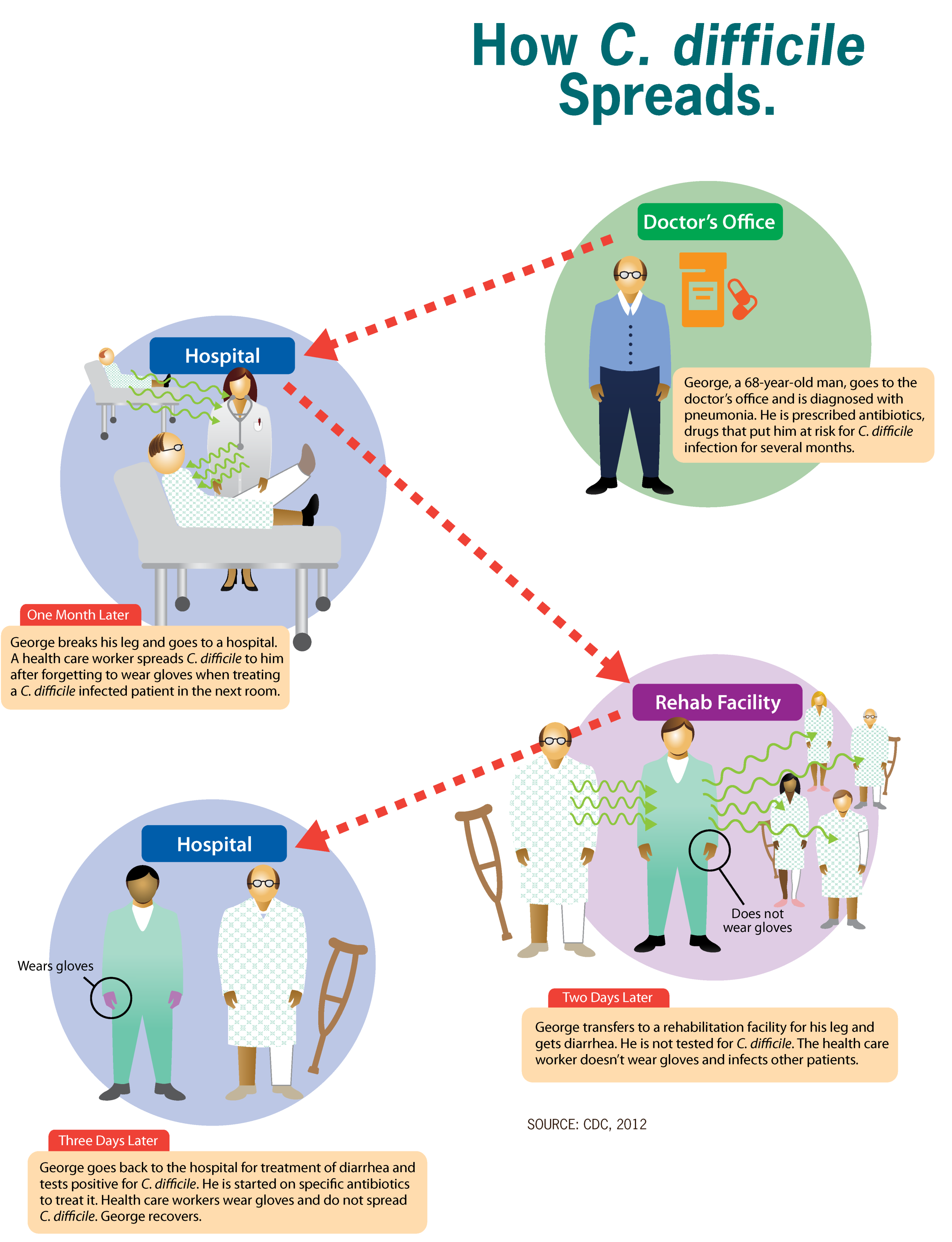
''Clostridioides difficile'' infection (CDI or C-diff), also known as ''Clostridium difficile'' infection, is a symptomatic infection due to the bacterial spores, spore-forming bacterium ''Clostridioides difficile''. Symptoms include watery diarrhea, fever, nausea, and abdominal pain. It makes up about 20% of cases of antibiotic-associated diarrhea. Antibiotics can contribute to detrimental changes in gut microbiota; specifically, they decrease short-chain fatty acid absorption, which results in osmotic, or watery, diarrhea. Complications may include colitis#Infectious, pseudomembranous colitis, toxic megacolon, perforation of the colon, and sepsis. ''Clostridioides difficile'' infection is spread by bacterial spores found within feces. Surfaces may become contaminated with the spores, with further spread occurring via the hands of healthcare workers. Risk factors for infection include antibiotic or proton pump inhibitor use, hospitalization, hypoalbuminemia, other health pro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Small Molecule
In molecular biology and pharmacology, a small molecule or micromolecule is a low molecular weight (≤ 1000 daltons) organic compound that may regulate a biological process, with a size on the order of 1 nm. Many drugs are small molecules; the terms are equivalent in the literature. Larger structures such as nucleic acids and proteins, and many polysaccharides are not small molecules, although their constituent monomers (ribo- or deoxyribonucleotides, amino acids, and monosaccharides, respectively) are often considered small molecules. Small molecules may be used as research tools to probe biological function as well as leads in the development of new therapeutic agents. Some can inhibit a specific function of a protein or disrupt protein–protein interactions. Pharmacology usually restricts the term "small molecule" to molecules that bind specific biological macromolecules and act as an effector, altering the activity or function of the target. Small molecules can ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phase II Trials
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants (potentially tens of thousands) to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays. Description Clinical trials testing potential medical products are commonly classified into four phases. The drug development process will normally proceed through all four phases over many years. When expressed specifically, a clinical trial phase is capitalized both in name and Roman numeral, such as "Phase I" clinical trial. If the drug successfully passes through Phases I, II, and III, it will usually be approved by the national regulatory auth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antibiotics
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy, treatment and antibiotic prophylaxis, prevention of such infections. They may either bactericide, kill or bacteriostatic agent, inhibit the growth of bacteria. A limited number of antibiotics also possess antiprotozoal activity. Antibiotics are not effective against viruses such as the ones which cause the common cold or influenza. Drugs which inhibit growth of viruses are termed antiviral drugs or antivirals. Antibiotics are also not effective against fungi. Drugs which inhibit growth of fungi are called antifungal drugs. Sometimes, the term ''antibiotic''—literally "opposing life", from the Greek language, Greek roots ἀντι ''anti'', "against" and βίος ''bios'', "life"—is broadly used to refer to any substance used against ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surotomycin
Surotomycin was an investigational oral antibiotic. This macrolide antibiotic was under investigation by Merck & Co (who acquired Cubist Pharmaceuticals) for the treatment of life-threatening diarrhea, commonly caused by the bacterium ''Clostridioides difficile''. After reaching phase III in clinical trials, its production was discontinued in 2017 due to its non-superiority to current therapies. See also * Cadazolid * Fidaxomicin * Ridinilazole Ridinilazole (previously known as SMT19969) is an investigational small molecule antibiotic under evaluation for oral administration for the treatment of Clostridioides difficile infection, ''Clostridioides difficile'' infection (CDI). In vitro, ... * SCHEMBL19952957 References Antibiotics Macrolides {{antibiotic-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SCHEMBL19952957
SCHEMBL19952957 is an oxadiazole based antibiotic, originally developed in 2014 as a potential treatment for infections with methicillin-resistant ''Staphylococcus aureus'' (MRSA) and other antibiotic-resistant bacteria. Subsequently, it has been found to be useful against ''Clostridioides difficile'' as it not only kills active bacteria but also inhibits the germination of the dormant spores which can otherwise often lead to persistent infections that repeatedly recur upon cessation of antibiotic treatment. While it is still only being researched in animals at this stage, this dual action is a significant advance over existing antibiotics, and it is likely that drugs from this class may be developed as new medications for the treatment of antibiotic-resistant infections in humans. See also * Cadazolid * Fidaxomicin * Ridinilazole * Surotomycin Surotomycin was an investigational oral antibiotic. This macrolide antibiotic was under investigation by Merck & Co (who acquired C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fidaxomicin
Fidaxomicin, sold under the brand name Dificid (by Merck) among others, is the first member of a class of narrow spectrum macrocyclic antibiotic drugs called tiacumicins. It is a fermentation product obtained from the actinomycete '' Dactylosporangium aurantiacum'' subspecies ''hamdenesis''. Fidaxomicin is minimally absorbed into the bloodstream when taken orally, is bactericidal, and selectively eradicates pathogenic ''Clostridioides difficile'' with relatively little disruption to the multiple species of bacteria that make up the normal, healthy intestinal microbiota. The maintenance of normal physiological conditions in the colon may reduce the probability of recurrence of ''Clostridioides difficile'' infection. It is marketed by Merck, which acquired Cubist Pharmaceuticals in 2015, and had in turn bought the originating company, Optimer Pharmaceuticals. It is used for the treatment of ''Clostridioides difficile'' infection, which is also known as ''Clostridioides dif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cadazolid
Cadazolid is an experimental antibiotic of the oxazolidinone class made by Actelion Pharmaceuticals Ltd. which is effective against ''Clostridioides difficile'', a major cause of drug resistant diarrhea in the elderly. Current drug treatments for this infection involve orally delivered antibiotics, principally fidaxomicin, metronidazole and vancomycin; the last two drugs are the principal therapeutic agents in use, but fail in approximately 20 to 45% of the cases. The drug works by inhibiting the synthesis of proteins in the bacteria, thus inhibiting the production of toxins and the formation of spores. Cadazolid progressed through to Phase III clinical trials, but in its financial results for Q1 2018, Idorsia mentions that Actelion informed them that "following completion of Phase 3 data analysis of cadazolid - it has decided to discontinue the development of the compound." __TOC__ Structure The chemical structure of cadazolid combines the pharmacophores of oxazolidinone and f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Food And Drug Administration
The United States Food and Drug Administration (FDA or US FDA) is a List of United States federal agencies, federal agency of the United States Department of Health and Human Services, Department of Health and Human Services. The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, caffeine products, dietary supplements, Prescription drug, prescription and Over-the-counter drug, over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood transfusions, medical devices, electromagnetic radiation emitting devices (ERED), cosmetics, Animal feed, animal foods & feed and Veterinary medicine, veterinary products. The FDA's primary focus is enforcement of the Federal Food, Drug, and Cosmetic Act (FD&C). However, the agency also enforces other laws, notably Section 361 of the Public Health Service Act as well as associated regulations. Much of this regulatory-enforcement work is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fast Track (FDA)
Fast track is a designation by the United States Food and Drug Administration (FDA) of an investigational drug for expedited review to facilitate development of drugs that treat a serious or life-threatening condition and fill an unmet medical need. Fast track designation must be requested by the drug company. The request can be initiated at any time during the drug development process. FDA will review the request and attempt to make a decision within sixty days. Purpose Fast track is one of five FDA approaches to make new drugs available as rapidly as possible: the others are priority review, breakthrough therapy, accelerated approval and regenerative medicine advanced therapy. Fast track was introduced by the FDA Modernization Act of 1997. Requirements Fast track designation is designed to aid in the development and expedite the review of drugs which show promise in treating a serious or life-threatening disease and address an unmet medical need. Serious condition: det ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Qualified Infectious Disease Product
The Food and Drug Administration Safety and Innovation Act of 2012 (FDASIA) is a piece of American regulatory legislation signed into law on July 9, 2012. It gives the United States Food and Drug Administration (FDA) the authority to collect user fees from the medical industry to fund reviews of innovator drugs, medical devices, generic drugs and biosimilar biologics. It also creates the breakthrough therapy designation program and extends the priority review voucher program to make eligible rare pediatric diseases. The measure was passed by 96 senators voting for and one voting against. Title I: Fees Relating to Drugs Title I extends through FY2017 the authority of the FDA, through the authority of the Secretary of Health and Human Services, to collect drug application and supplement fees, prescription drug establishment fees, and prescription drug product fees to support the FDA process for reviewing human drug applications. It requires the FDA to submit annually to the Hou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fidaxomicin
Fidaxomicin, sold under the brand name Dificid (by Merck) among others, is the first member of a class of narrow spectrum macrocyclic antibiotic drugs called tiacumicins. It is a fermentation product obtained from the actinomycete '' Dactylosporangium aurantiacum'' subspecies ''hamdenesis''. Fidaxomicin is minimally absorbed into the bloodstream when taken orally, is bactericidal, and selectively eradicates pathogenic ''Clostridioides difficile'' with relatively little disruption to the multiple species of bacteria that make up the normal, healthy intestinal microbiota. The maintenance of normal physiological conditions in the colon may reduce the probability of recurrence of ''Clostridioides difficile'' infection. It is marketed by Merck, which acquired Cubist Pharmaceuticals in 2015, and had in turn bought the originating company, Optimer Pharmaceuticals. It is used for the treatment of ''Clostridioides difficile'' infection, which is also known as ''Clostridioides dif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |