|
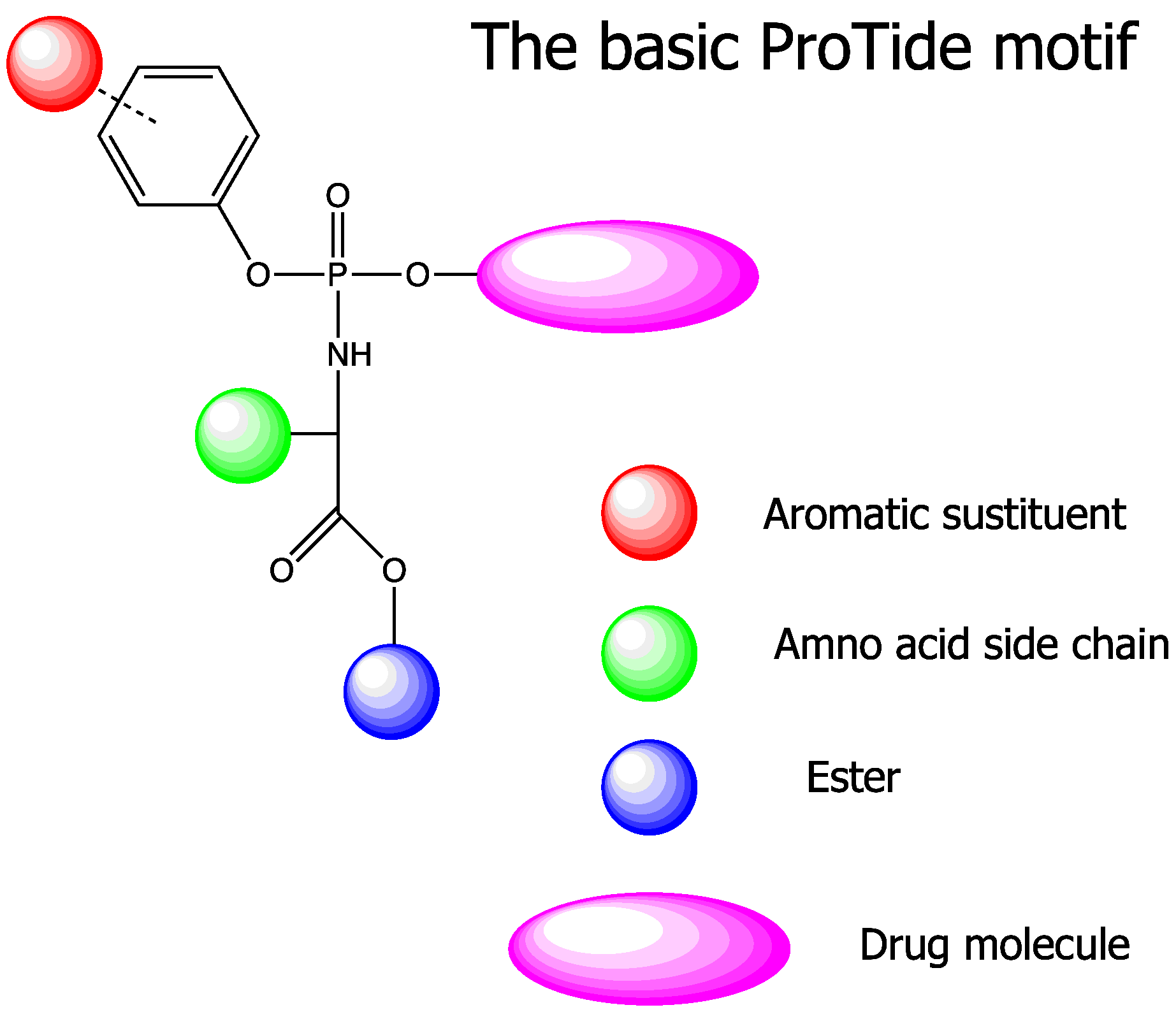
ProTide
The ProTide technology is a prodrug approach used in molecular biology and drug design. It is designed to deliver nucleotide analogues (as monophosphate) into the cell (ProTide: PROdrug + nucleoTIDE). This technology was invented by Professor Chris McGuigan from the School of Pharmacy and Pharmaceutical Sciences at Cardiff University in the early 1990s. ProTides form a critical part of antiviral drugs such as sofosbuvir, tenofovir alafenamide, and remdesivir. __TOC__ Development The first demonstration of the ProTide approach was made in 1992, when the efficiency of aryloxy phosphates and phosphoramidates was noted. In particular, diaryl phosphates were prepared from zidovudine (AZT) using simple phosphorochloridate chemistry. For the first time, the anti-HIV activity of these phosphate derivatives of AZT exceeded that of the parent nucleoside in some cases. Moreover, while AZT was almost inactive ( EC50 100μM) in the JM cell line, the substituted diaryl phosphate was 10 tim ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sofosbuvir
Sofosbuvir, sold under the brand name Sovaldi among others, is a medication used to treat hepatitis C. It is taken by mouth. Common side effects include fatigue, headache, nausea, and trouble sleeping. Side effects are generally more common in interferon-containing regimens. Sofosbuvir may reactivate hepatitis B in those who have been previously infected. In combination with ledipasvir, daclatasvir or simeprevir, it is not recommended with amiodarone due to the risk of an abnormally slow heartbeat. Sofosbuvir is in the nucleotide analog family of medications and works by blocking the hepatitis C NS5B protein. Sofosbuvir was discovered in 2007 and approved for medical use in the United States in 2013. It is on the World Health Organization's List of Essential Medicines. Medical uses Initial HCV treatment In 2016, the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America jointly published a recommendation for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amino Acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 appear in the genetic code of life. Amino acids can be classified according to the locations of the core structural functional groups ( alpha- , beta- , gamma- amino acids, etc.); other categories relate to polarity, ionization, and side-chain group type ( aliphatic, acyclic, aromatic, polar, etc.). In the form of proteins, amino-acid '' residues'' form the second-largest component (water being the largest) of human muscles and other tissues. Beyond their role as residues in proteins, amino acids participate in a number of processes such as neurotransmitter transport and biosynthesis. It is thought that they played a key role in enabling life on Earth and its emergence. Amino acids are formally named by the IUPAC- IUBMB Joint Commi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carboxylesterase 1
Liver carboxylesterase 1 also known as carboxylesterase 1 (CES1, hCE-1 or CES1A1) is an enzyme that in humans is encoded by the ''CES1'' gene. The protein is also historically known as serine esterase 1 (SES1), monocyte esterase and cholesterol ester hydrolase (CEH). Three transcript variants encoding three different isoforms have been found for this gene. The various protein products from isoform a, b and c range in size from 568, 567 and 566 amino acids long, respectively. CES1 is present in most tissues with higher levels in the liver and low levels in the gastrointestinal tract. Function Carboxylesterase 1 is a serine esterase and member of a large multigene carboxylesterase family. It is also part of the alpha/beta hydrolase fold, alpha/beta fold hydrolase family. These enzymes are responsible for the hydrolysis of ester- and amide-bond-containing xenobiotics and drugs such as cocaine and heroin. They also hydrolyze long-chain fatty acid esters and thioesters. As part of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cathepsin A
Cathepsin A is an enzyme that is classified both as a cathepsin and a carboxypeptidase. In humans, it is encoded by the ''CTSA'' gene. The enzyme is also known as Human Protective Protein. It is a lysosomal serine carboxypeptidase. The enzyme is a zymogen and must be processed to produce a 32 kDa and 20 kDa large and small subunit, respectively, to become catalytically active. Cathespin L can activate Cathepsin A in vitro. Structure Cathepsin A contains a large and small subunit. The active site contains unusual pairs of carboxylic acids hydrogen bonded to one another, sometimes referred to as "Rebek pairs". The pairing of these carboxylic acids raises the pKa of one glutamate to ~13 while the other has a predicted pKa of ~6. Function This gene encodes a glycoprotein that associates with lysosomal enzymes beta-galactosidase and neuraminidase to form a complex of high-molecular-weight multimers. The formation of this complex provides a protective role for stability and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phosphonate
In organic chemistry, phosphonates or phosphonic acids are organophosphorus compounds containing Functional group, groups, where R is an organic group (alkyl, aryl). If R is hydrogen then the compound is a Phosphite_ester#Chemistry_of_HP(O)(OR)2, dialkyl phosphite, which is a different functional group. Phosphonic acids, typically handled as salts, are generally Volatility (chemistry), nonvolatile solids that are poorly soluble in organic solvents, but soluble in water and common Alcohol (chemistry), alcohols. Many commercially important compounds are phosphonates, including glyphosate (the active molecule of the herbicide Roundup (herbicide), Roundup), and ethephon, a widely used plant growth regulator. Bisphosphonates are popular drugs for treatment of osteoporosis.Svara, J.; Weferling, N.; Hofmann, T. "Phosphorus Compounds, Organic," in ''Ullmann's Encyclopedia of Industrial Chemistry'', Wiley-VCH, Weinheim, 2008. . In biochemistry and medicinal chemistry, phosphonate gr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stavudine
Stavudine (d4T), sold under the brand name Zerit among others, is an antiretroviral medication used to prevent and treat HIV/AIDS. It is generally recommended for use with other antiretrovirals. It may be used for prevention after a needlestick injury or other potential exposure. However, it is not a first-line treatment. It is given by mouth. Common side effects include headache, diarrhea, vomiting, rash, and peripheral nerve problems. Severe side effects include high blood lactate, pancreatitis, and an enlarged liver. It is not generally recommended in pregnancy. Stavudine is in the nucleoside analog reverse-transcriptase inhibitor (NRTI) class of medication. Stavudine was first described in 1966 and approved for use in the United States in 1994. It is available as a generic medication. Medical uses Stavudine is used in the treatment of HIV-1 infection, but is not a cure. It is not normally recommended as initial treatment. Stavudine can also reduce the risk of devel ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenylalanine
Phenylalanine (symbol Phe or F) is an essential α-amino acid with the chemical formula, formula . It can be viewed as a benzyl group substituent, substituted for the methyl group of alanine, or a phenyl group in place of a terminal hydrogen of alanine. This essential amino acid is classified as neutral, and chemical polarity, nonpolar because of the inert and hydrophobic nature of the benzyl side chain. The chirality (chemistry)#Naming conventions, L-isomer is used to biochemically form proteins coded for by DNA. Phenylalanine is a precursor for tyrosine, the monoamine neurotransmitters dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline), and the biological pigment melanin. It is Genetic code, encoded by the messenger RNA codons UUU and UUC. Phenylalanine is found naturally in the milk of mammals. It is used in the manufacture of food and drink products and sold as a nutritional supplement as it is a direct precursor to the neuromodulation, neuromodulator phe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycine
Glycine (symbol Gly or G; ) is an amino acid that has a single hydrogen atom as its side chain. It is the simplest stable amino acid. Glycine is one of the proteinogenic amino acids. It is encoded by all the codons starting with GG (GGU, GGC, GGA, GGG). Glycine disrupts the formation of alpha-helices in secondary protein structure. Its small side chain causes it to favor random coils instead. Glycine is also an inhibitory neurotransmitter – interference with its release within the spinal cord (such as during a '' Clostridium tetani'' infection) can cause spastic paralysis due to uninhibited muscle contraction. It is the only achiral proteinogenic amino acid. It can fit into both hydrophilic and hydrophobic environments, due to its minimal side chain of only one hydrogen atom. History and etymology Glycine was discovered in 1820 by French chemist Henri Braconnot when he hydrolyzed gelatin by boiling it with sulfuric acid. He originally called it "sugar of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
HIV-2
The subtypes of HIV include two main subtypes, known as HIV type 1 (HIV-1) and HIV type 2 (HIV-2). These subtypes have distinct genetic differences and are associated with different epidemiological patterns and clinical characteristics. HIV-1 exhibits a genetic relation to viruses indigenous to chimpanzees and gorillas that inhabit West Africa, while HIV-2 viruses are affiliated with viruses present in the sooty mangabey, a vulnerable West African primate. HIV-1 viruses can be further stratified into groups M, N, O, and P. Among these, HIV-1 group M viruses are the most prevalent, infecting nearly 90% of people living with HIV and are responsible for the global AIDS pandemic. Group M can be further subdivided into subtypes based on genetic sequence data. Certain subtypes are known for their increased virulence or drug resistance to different medications used to treat HIV. HIV-2 viruses are generally considered to be less virulent and less transmissible than HIV-1 M group vir ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thymidine Kinase
Thymidine kinase is an enzyme, a phosphotransferase (a kinase): 2'-deoxythymidine kinase, ATP-thymidine 5'-phosphotransferase, EC 2.7.1.21. It can be found in most living cells. It is present in two forms in mammalian cells, TK1 and TK2. Certain viruses also have genetic information for expression of viral thymidine kinases. Thymidine kinase catalyzes the reaction: :Thd + ATP → TMP + ADP where Thd is (deoxy)thymidine, ATP is adenosine triphosphate, TMP is (deoxy) thymidine monophosphate and ADP is adenosine diphosphate. Thymidine kinases have a key function in the synthesis of DNA and therefore in cell division, as they are part of the unique reaction chain to introduce thymidine into the DNA. Thymidine is present in the body fluids as a result of degradation of DNA from food and from dead cells. Thymidine kinase is required for the action of many antiviral drugs. It is used to select hybridoma cell lines in production of monoclonal antibodies. In clinical chemistry it is u ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |