|
Protein Electrophoresis
Protein electrophoresis is a method for analysing the proteins in a fluid or an extract. The electrophoresis may be performed with a small volume of sample in a number of alternative ways with or without a supporting medium, namely agarose or polyacrylamide. Variants of gel electrophoresis include SDS-PAGE, free-flow electrophoresis, electrofocusing, isotachophoresis, affinity electrophoresis, immunoelectrophoresis, counterelectrophoresis, and capillary electrophoresis. Each variant has many subtypes with individual advantages and limitations. Gel electrophoresis is often performed in combination with electroblotting or immunoblotting to give additional information about a specific protein. Denaturing gel methods SDS-PAGE SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis, describes a collection of related techniques to separate proteins according to their Electrophoresis, electrophoretic mobility (a function of the molecular weight of a polypeptide chain) while ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrophoresis
Electrophoresis is the motion of charged dispersed particles or dissolved charged molecules relative to a fluid under the influence of a spatially uniform electric field. As a rule, these are zwitterions with a positive or negative net charge. Electrophoresis is used in laboratories to separate macromolecules based on their charges. The technique normally applies a negative charge called cathode so anionic protein molecules move towards a positive charge called anode. Therefore, electrophoresis of positively charged particles or molecules (cations) is sometimes called cataphoresis, while electrophoresis of negatively charged particles or molecules (anions) is sometimes called anaphoresis. Electrophoresis is the basis for analytical techniques used in biochemistry and molecular biology to separate particles, molecules, or ions by size, charge, or binding affinity, either freely or through a supportive medium using a one-directional flow of electrical charge. It is use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prosthetic Group
A prosthetic group is the non-amino acid component that is part of the structure of the heteroproteins or conjugated proteins, being tightly linked to the apoprotein. Not to be confused with the cosubstrate that binds to the enzyme apoenzyme (either a holoprotein or heteroprotein) by non-covalent binding a non-protein (non-amino acid) This is a component of a conjugated protein that is required for the protein's biological activity. The prosthetic group may be organic (such as a vitamin, sugar, RNA, phosphate or lipid) or inorganic (such as a metal ion). Prosthetic groups are bound tightly to proteins and may even be attached through a covalent bond. They often play an important role in enzyme catalysis. A protein without its prosthetic group is called an apoprotein, while a protein combined with its prosthetic group is called a holoprotein. A non-covalently bound prosthetic group cannot generally be removed from the holoprotein without denaturating the protein. Thus, the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorescence
Fluorescence is one of two kinds of photoluminescence, the emission of light by a substance that has absorbed light or other electromagnetic radiation. When exposed to ultraviolet radiation, many substances will glow (fluoresce) with colored visible light. The color of the light emitted depends on the chemical composition of the substance. Fluorescent materials generally cease to glow nearly immediately when the radiation source stops. This distinguishes them from the other type of light emission, phosphorescence. Phosphorescent materials continue to emit light for some time after the radiation stops. This difference in duration is a result of quantum spin effects. Fluorescence occurs when a photon from incoming radiation is absorbed by a molecule, exciting it to a higher energy level, followed by the emission of light as the molecule returns to a lower energy state. The emitted light may have a longer wavelength and, therefore, a lower photon energy than the absorbed radi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
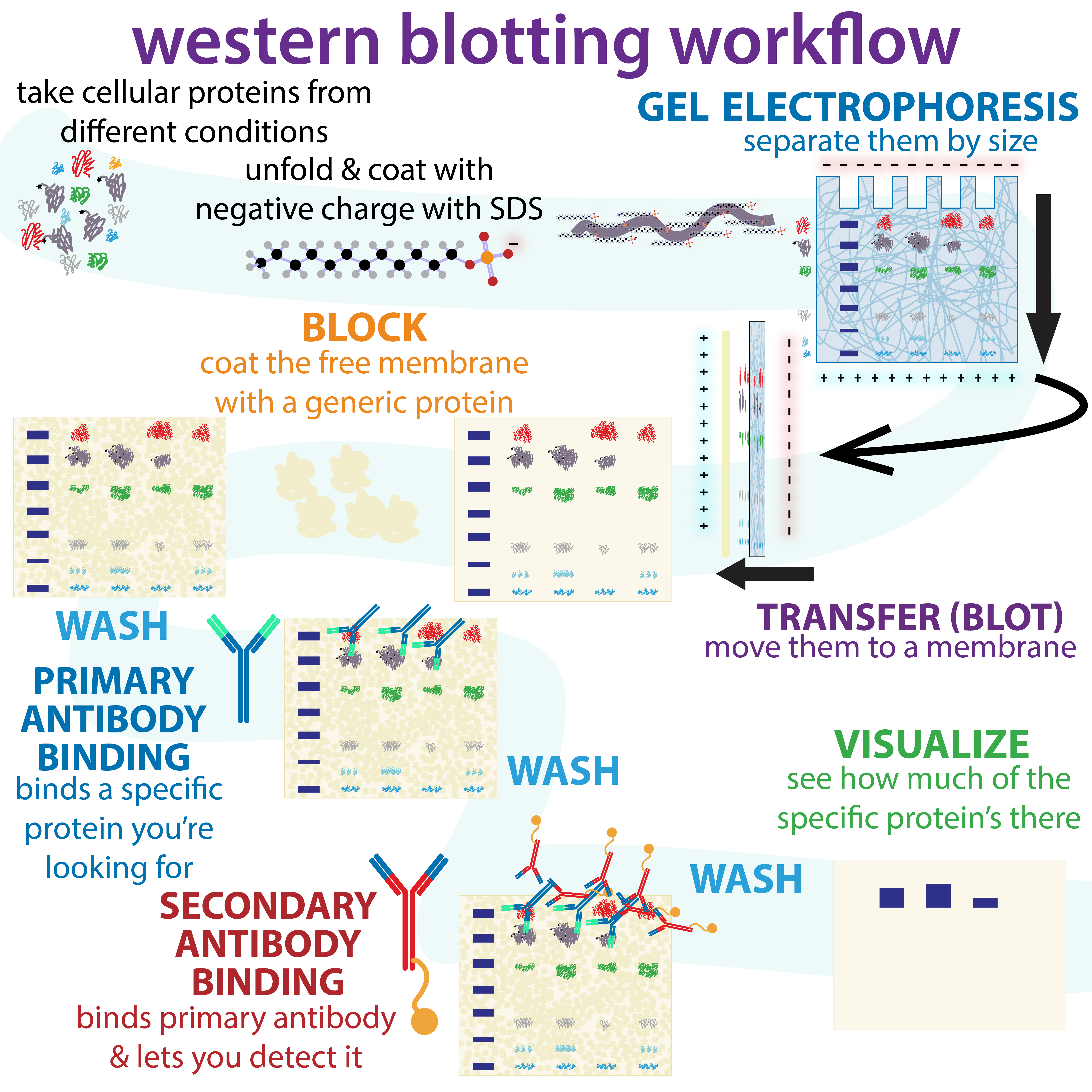
Western Blot
The western blot (sometimes called the protein immunoblot), or western blotting, is a widely used analytical technique in molecular biology and immunogenetics to detect specific proteins in a sample of tissue homogenate or extract. Besides detecting the proteins, this technique is also utilized to visualize, distinguish, and quantify the different proteins in a complicated protein combination. Western blot technique uses three elements to achieve its task of separating a specific protein from a complex: separation by size, transfer of protein to a solid support, and marking target protein using a primary and secondary antibody to visualize. A synthetic or animal-derived antibody (known as the primary antibody) is created that recognizes and binds to a specific target protein. The electrophoresis membrane is washed in a solution containing the primary antibody, before excess antibody is washed off. A secondary antibody is added which recognizes and binds to the primary antibod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemoluminescence
Chemiluminescence (also chemoluminescence) is the emission of light (luminescence) as the result of a chemical reaction, i.e. a chemical reaction results in a flash or glow of light. A standard example of chemiluminescence in the laboratory setting is the luminol test. Here, blood is indicated by luminescence upon contact with iron in hemoglobin. When chemiluminescence takes place in living organisms, the phenomenon is called bioluminescence. A light stick emits light by chemiluminescence. Physical description As in many chemical reactions, chemiluminescence starts with the combining of two compounds, say A and B, to give a product C. Unlike most chemical reactions, the product C converts to a further product, which is produced in an electronically excited state often indicated with an asterisk: : A + B → C : C → D* D* then emits a photon (''h''ν), to give the ground state of D: I : D* → D + ''h''ν In theory, one photon of light should be given off for each mo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quenching (fluorescence)
In chemistry, quenching refers to any process which decreases the fluorescent intensity of a given substance. A variety of processes can result in quenching, such as excited state reactions, energy transfer, complex-formation and collisions. As a consequence, quenching is often heavily dependent on pressure and temperature. Molecular oxygen, iodine ions and acrylamide are common chemical quenchers. The chloride ion is a well known quencher for quinine fluorescence. Quenching poses a problem for non-instant spectroscopic methods, such as laser-induced fluorescence. Quenching is made use of in optode sensors; for instance the quenching effect of oxygen on certain ruthenium complexes allows the measurement of oxygen saturation in solution. Quenching is the basis for Förster resonance energy transfer (FRET) assays. Quenching and dequenching upon interaction with a specific molecular biological target is the basis for activatable optical contrast agents for molecular imaging. M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dissociate
Dissociation in chemistry is a general process in which molecules (or ionic compounds such as salts, or complexes) separate or split into other things such as atoms, ions, or radicals, usually in a reversible manner. For instance, when an acid dissolves in water, a covalent bond between an electronegative atom and a hydrogen atom is broken by heterolytic fission, which gives a proton (H+) and a negative ion. Dissociation is the opposite of association or recombination. Dissociation constant For reversible dissociations in a chemical equilibrium :AB A + B the dissociation constant ''K''d is the ratio of dissociated to undissociated compound :K_d = \mathrm where the brackets denote the equilibrium concentrations of the species. Dissociation degree The dissociation degree \alpha is the fraction of original solute molecules that have dissociated. It is usually indicated by the Greek symbol α. More accurately, degree of dissociation refers to the amount of solute dissocia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Charge (physics)
In physics, a charge is any of many different quantities, such as the electric charge in electromagnetism or the color charge in quantum chromodynamics. Charges correspond to the time-invariant generators of a symmetry group, and specifically, to the generators that commute with the Hamiltonian. Charges are often denoted by Q, and so the invariance of the charge corresponds to the vanishing commutator ,H0, where H is the Hamiltonian. Thus, charges are associated with conserved quantum numbers; these are the eigenvalues of the generator Q. A "charge" can also refer to a point-shaped object with an electric charge and a position, such as in the method of image charges. Abstract definition Abstractly, a charge is any generator of a continuous symmetry of the physical system under study. When a physical system has a symmetry of some sort, Noether's theorem implies the existence of a conserved current. The thing that "flows" in the current is the "charge"; the charge is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coomassie Brilliant Blue
Coomassie brilliant blue is the name of two similar triphenylmethane dyes that were developed for use in the textile industry but are now commonly used for staining proteins in analytical biochemistry. Coomassie brilliant blue G-250 differs from Coomassie brilliant blue R-250 by the addition of two methyl groups. The name "Coomassie" is a registered trademark of Imperial Chemical Industries. Name and discovery The name Coomassie was adopted at the end of the 19th century as a trade name by the Blackley-based dye manufacturer Levinstein Ltd, in marketing a range of acid wool dyes. In 1896 during the Fourth Anglo–Ashanti War, British forces had occupied the town of Coomassie (modern-day Kumasi in Ghana). In 1918 Levinstein Ltd became part of British Dyestuffs, which in 1926 became part of Imperial Chemical Industries. Although ICI still owns the Coomassie trademark, the company no longer manufactures the dyes. The blue disulfonated triphenylmethane dyes were first produced ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PAGE
Page most commonly refers to: * Page (paper), one side of a leaf of paper, as in a book Page, PAGE, pages, or paging may also refer to: Roles * Page (assistance occupation), a professional occupation * Page (servant), traditionally a young male servant * Page (wedding attendant) People and fictional characters * Page (given name), a list of people * Page (surname), a list of people and fictional characters * Pages (surname) * H. A. Page, a pen name of Scottish author Alexander Hay Japp (1836–1905) Places Australia * Page, Australian Capital Territory, a suburb of Canberra * Division of Page, New South Wales * Pages River, a tributary of the Hunter River catchment in New South Wales, Australia * The Pages, South Australia, two islands and a reef ** The Pages Conservation Park, a protected area in South Australia United States * Page, Arizona, a city * Page, Indiana * Page, Minneapolis, Minnesota, a neighborhood * Page, Nebraska, a village * Page, North Dakota, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Logarithm
In mathematics, the logarithm of a number is the exponent by which another fixed value, the base, must be raised to produce that number. For example, the logarithm of to base is , because is to the rd power: . More generally, if , then is the logarithm of to base , written , so . As a single-variable function, the logarithm to base is the inverse of exponentiation with base . The logarithm base is called the ''decimal'' or ''common'' logarithm and is commonly used in science and engineering. The ''natural'' logarithm has the number as its base; its use is widespread in mathematics and physics because of its very simple derivative. The ''binary'' logarithm uses base and is widely used in computer science, information theory, music theory, and photography. When the base is unambiguous from the context or irrelevant it is often omitted, and the logarithm is written . Logarithms were introduced by John Napier in 1614 as a means of simplifying calculation ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |