|
Potassium Hexafluoronickelate(IV)
Potassium hexafluoronickelate(IV) is an inorganic compound with the chemical formula . It can be produced through the reaction of potassium fluoride, nickel dichloride, and fluorine. It reacts violently with water, releasing oxygen. It dissolves in anhydrous hydrogen fluoride to produce a light-red solution. Potassium hexafluoronickelate(IV) decomposes at 350 °C, forming potassium hexafluoronickelate(III), nickel(II) fluoride, and fluorine: :\rm \ 3 K_2NiF_6 \xrightarrow 2 K_3NiF_6 + NiF_2 + 2 F_2 Potassium hexafluoronickelate is a strong oxidant. It can turn chlorine pentafluoride and bromine pentafluoride Bromine pentafluoride, Br F5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent. BrF5 finds use in oxygen isotope analysis. Laser ablation of solid silicates in the presence of BrF5 releases O2 for subs ... into and , respectively: :\rm \ K_2NiF_6 + 5 AsF_5 + XF_5 \xrightarrow XF_6AsF_6 + Ni(AsF_6)_2 + 2KAsF_6 :( X = ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
American Elements
American Elements is a global manufacturer and distributor of advanced materials with an over 35,000-page online product catalog and compendium of information on the chemical elements, advanced materials, and high technology applications. The company's headquarters and educational programs are based in Los Angeles, California. Its research and production facilities are located in Salt Lake City, Utah; Monterrey, Mexico;China; and Manchester, UK. History American Elements began as a toll chemical manufacturer and refiner serving U.S. mining companies by producing metal-based chemicals from their deposits. In 1998, its two largest customers, the Unocal/Molycorp rare-earth mine in Mountain Pass, California, and the Rhodia rare-earth refinery in Freeport, Texas closed, ending domestic U.S. rare-earth production. In response, the company established mining joint ventures in Inner Mongolia, China and in 1999 became one of the first post-Cold War companies to export rare-earth metals ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
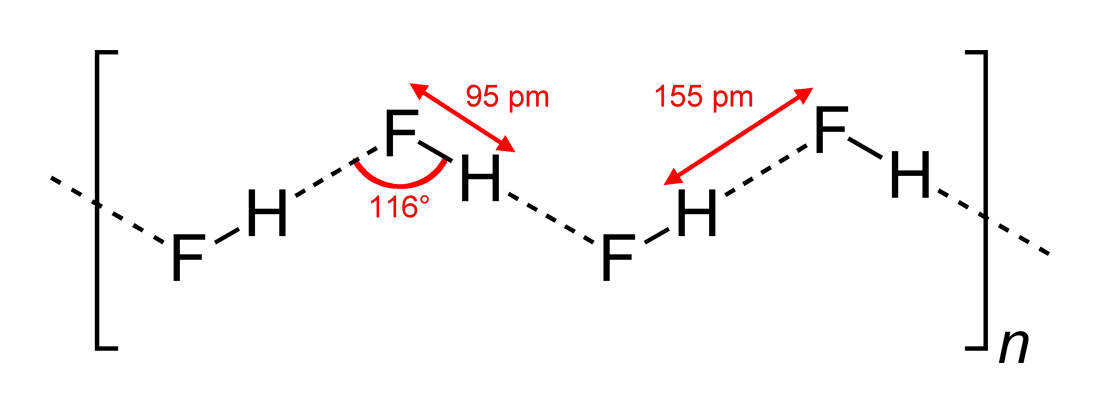
Potassium Fluoride
Potassium fluoride is the chemical compound with the formula KF. After hydrogen fluoride, KF is the primary source of the fluoride ion for applications in manufacturing and in chemistry. It is an alkali halide salt and occurs naturally as the rare mineral carobbiite. Solutions of KF will etch glass due to the formation of soluble fluorosilicates, although HF is more effective. Preparation Potassium fluoride is prepared by reacting potassium carbonate with hydrofluoric acid. Evaporation of the solution forms crystals of potassium bifluoride. The bifluoride on heating yields potassium fluoride: : : Platinum or heat resistant plastic containers are often used for these operations. Potassium chloride converts to KF upon treatment with hydrogen fluoride. In this way, potassium fluoride is recyclable. Crystalline properties KF crystallizes in the cubic NaCl crystal structure. The lattice parameter at room temperature is 0.266 nm. Applications in organic chemistry In organic c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nickel Dichloride
Nickel(II) chloride (or just nickel chloride) is the chemical compound NiCl2. The anhydrous salt (chemistry), salt is yellow, but the more familiar Water of crystallization, hydrate NiCl2·6H2O is green. Nickel(II) chloride, in various forms, is the most important source of nickel for chemical synthesis. The nickel chlorides are deliquescent, absorbing moisture from the air to form a solution. Nickel salts have been shown to be carcinogenic to the lungs and nasal passages in cases of long-term inhalation exposure. Production and syntheses Large scale production and uses of nickel chloride are associated with the purification of nickel from its ores. It is generated upon extraction nickel matte (metallurgy), matte and residues obtained from roasting refining nickel-containing ores using hydrochloric acid. Electrolysis of nickel chloride solutions are used in the production of nickel metal. Other significant routes to nickel chloride arise from processing of ore concentrates such ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Fluorine
Fluorine is a chemical element; it has Chemical symbol, symbol F and atomic number 9. It is the lightest halogen and exists at Standard temperature and pressure, standard conditions as pale yellow Diatomic molecule, diatomic gas. Fluorine is extremely Reactivity (chemistry), reactive as it reacts with all other Periodic table, elements except for the light Noble gas, noble gases. It is highly toxicity, toxic. Among the elements, fluorine ranks Abundance of the chemical elements, 24th in cosmic abundance and 13th in crustal abundance. Fluorite, the primary mineral source of fluorine, which gave the element its name, was first described in 1529; as it was added to metal ores to lower their melting points for smelting, the Latin verb meaning gave the mineral its name. Proposed as an element in 1810, fluorine proved difficult and dangerous to separate from its compounds, and several early experimenters died or sustained injuries from their attempts. Only in 1886 did French chemist He ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nickel(II) Fluoride
Nickel(II) fluoride is the chemical compound with the formula NiF2. It is an ionic compound of nickel and fluorine and forms yellowish to green tetragonal crystals. Unlike many fluorides, NiF2 is stable in air. Nickel(II) fluoride is also produced when nickel metal is exposed to fluorine. In fact, NiF2 comprises the passivating surface that forms on nickel alloys (e.g. monel) in the presence of hydrogen fluoride or elemental fluorine. For this reason, nickel and its alloys are suitable materials for storage and transport these fluorine and related fluorinating agents. NiF2 is also used as a catalyst for the synthesis of chlorine pentafluoride. Preparation and structure NiF2 is prepared by treatment of anhydrous nickel(II) chloride with fluorine at 350 °C: :NiCl2 + F2 → NiF2 + Cl2 The corresponding reaction of cobalt(II) chloride results in oxidation of the cobalt, whereas nickel remains in the +2 oxidation state after fluorination because its +3 oxidation state i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Mg2FeH6 From X-ray
MG, Mg, or mg and variants may refer to: Arts Entertainment * MG, a character in ''The Perhapanauts'' comics * Magilla Gorilla, a cartoon character * ''Match Game'', a television game show Music * ''Main gauche'', "left hand" in piano playing * ''MG'' (album), a 2015 album by Martin Gore * The M.G.'s, from the band Booker T. & the M.G.'s * ''The MG's'' (album), an album by the M.G.'s * M:G, real name Maribel Gonzalez, dance music singer * MG Select, a house duo music production including George Jackson Military * Machine gun (MG-), prefix for model designations, for example, "MG42" * Major general, a military rank * Medal for Gallantry, a military decoration Organizations * MG Cars, an automotive marque of the now defunct MG Car Company * MG Motor, a present-day car manufacturing company ** JSW MG Motor India, Indian subsidiary of MG Motor ** MG Motors Pakistan, Pakistani subsidiary of MG Motor * Champion Air (IATA code) * Matematička gimnazija, a school in Belgrade * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Chlorine Pentafluoride
Chlorine pentafluoride is an interhalogen compound with formula . This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry, as confirmed by its high-resolution 19F NMR spectrum. It was first synthesized in 1963. Preparation Some of the earliest research on the preparation was classified. It was first prepared by fluorination of chlorine trifluoride at high temperatures and high pressures: : : : : catalyzes this reaction. Certain metal fluorides, , e.g. (potassium tetrafluorochlorate(III)), (rubidium tetrafluorochlorate(III)), (caesium tetrafluorochlorate(III)), react with to produce and the corresponding alkali metal fluoride. Reactions In a highly exothermic reaction, reacts with water to produce chloryl fluoride and hydrogen fluoride: : It is also a strong fluorinating agent. At room temperature it reacts readily with all elements (including otherwise "inert" elem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Bromine Pentafluoride
Bromine pentafluoride, Br F5, is an interhalogen compound and a fluoride of bromine. It is a strong fluorinating agent. BrF5 finds use in oxygen isotope analysis. Laser ablation of solid silicates in the presence of BrF5 releases O2 for subsequent analysis. It has also been tested as an oxidizer in liquid rocket propellants and is used as a fluorinating agent in the processing of uranium. Preparation BrF5 was first prepared in 1931 by the direct reaction of bromine and fluorine. This reaction is suitable for the preparation of large quantities, and is carried out at temperatures over with an excess of fluorine: :Br2 + 5 F2 → 2 BrF5 For the preparation of smaller amounts, potassium bromide is used: :KBr + 3 F2 → KF + BrF5 This route yields BrF5 almost completely free of trifluorides and other impurities. Reactions BrF5 reacts with water to form bromic acid and hydrofluoric acid: :BrF5 + 3 H2O → HBrO3 + 5 HF It is an extremely effective fluorinating agent, bei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Terbium(IV) Fluoride
Terbium is a chemical element; it has Symbol (chemistry), symbol Tb and atomic number 65. It is a silvery-white, rare earth element, rare earth metal that is malleable and ductile. The ninth member of the lanthanide series, terbium is a fairly electropositive metal that reacts with water, evolving hydrogen gas. Terbium is never found in nature as a free element, but it is contained in many minerals, including cerite, gadolinite, monazite, xenotime and euxenite. Swedish chemist Carl Gustaf Mosander discovered terbium as a chemical element in 1843. He detected it as an impurity in Yttrium(III) oxide, yttrium oxide (). Yttrium and terbium, as well as erbium and ytterbium, are named after the village of Ytterby in Sweden. Terbium was not isolated in pure form until the advent of ion exchange techniques. Terbium is used to dopant, dope calcium fluoride, calcium tungstate and strontium molybdate in solid-state devices, and as a crystal stabilizer of fuel cells that operate at elevated t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |