|
Polyyne
A polyyne is any organic compound with alternating Single bond, single and triple bonds; that is, a series of consecutive alkynes, with ''n'' greater than 1. These compounds are also called polyacetylenes, especially in the natural products and chemical ecology literature, even though this nomenclature more properly refers to acetylene polymers composed of alternating single and double bonds with ''n'' greater than 1. They are also sometimes referred to as oligoynes, or carbinoids after "linear acetylenic carbon, carbyne" , the hypothetical allotrope of carbon that would be the ultimate member of the series. In ''Avancés récentes en chimie des acétylènes – Recent advances in acetylene chemistry'' The synthesis of this substance has been claimed several times since the 1960s, but those reports have been disputed. Indeed, the substances identified as short chains of "carbyne" in many early organic synthesis attempts would be called polyynes today. The simplest polyyne is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Linear Acetylenic Carbon
Linear acetylenic carbon (LAC), also known as carbyne or a Linear Carbon Chain (LCC), is an Allotropes of carbon, allotrope of carbon that has the chemical structure as a repeat unit, with alternating Single bond, single and triple bonds. It would thus be the ultimate member of the polyyne family. This polymeric carbyne is of considerable interest to nanotechnology as its Young's modulus is – forty times that of diamond; this extraordinary number is, however, based on a novel definition of cross-sectional area that does not correspond to the space occupied by the structure. Carbyne has also been identified in interstellar space; however, its existence in condensed phases has been contested recently, as such chains would crosslink exothermically (and perhaps explosively) if they approached each other. History and controversy The first claims of detection of this allotrope were made in 1960Sladkov A.M, Kudryavtsev Y.P Diamond, graphite, carbyne 3/4 the allotropic forms of car ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Copper(I) Acetylide
Copper(I) acetylide, copper carbide or cuprous acetylide, is a chemical compound with the formula . It is a copper(I) salt of acetylene. It consists of cations and acetylide anions , with the triple bond between the two carbon atoms. Although never characterized by X-ray crystallography, the material has been claimed at least since 1856. One form is claimed to be a monohydrate with formula . Copper(I) acetylide is a reddish-brown explosive powder. Synthesis Materials purported to be copper acetylide can be prepared by treating acetylene with a solution of copper(I) chloride and ammonia: : This reaction produces a reddish solid precipitate. Properties When dry, copper acetylide is a heat and shock sensitive primary explosive, more sensitive than silver acetylide. In acetylene manufacturing plants, copper acetylide is thought to form inside pipes made of copper or an alloy with high copper content, which may result in violent explosion. This led to abandonment of copper as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polyacetylene
Polyacetylene (IUPAC name: polyethyne) usually refers to an organic polymer with the repeating unit . The name refers to its conceptual construction from polymerization of acetylene to give a chain with repeating olefin groups. This compound is conceptually important, as the discovery of polyacetylene and its high Ionic conductivity (solid state), conductivity upon doping (semiconductor), doping helped to launch the field of organic conductive polymers. The high electrical conductivity discovered by Hideki Shirakawa, Alan Heeger, and Alan MacDiarmid for this polymer led to intense interest in the use of organic compounds in microelectronics (organic semiconductors). This discovery was recognized by the Nobel Prize in Chemistry in 2000. Early work in the field of polyacetylene research was aimed at using doped polymers as easily processable and lightweight "plastic metals". Despite the promise of this polymer in the field of conductive polymers, many of its properties such as insta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
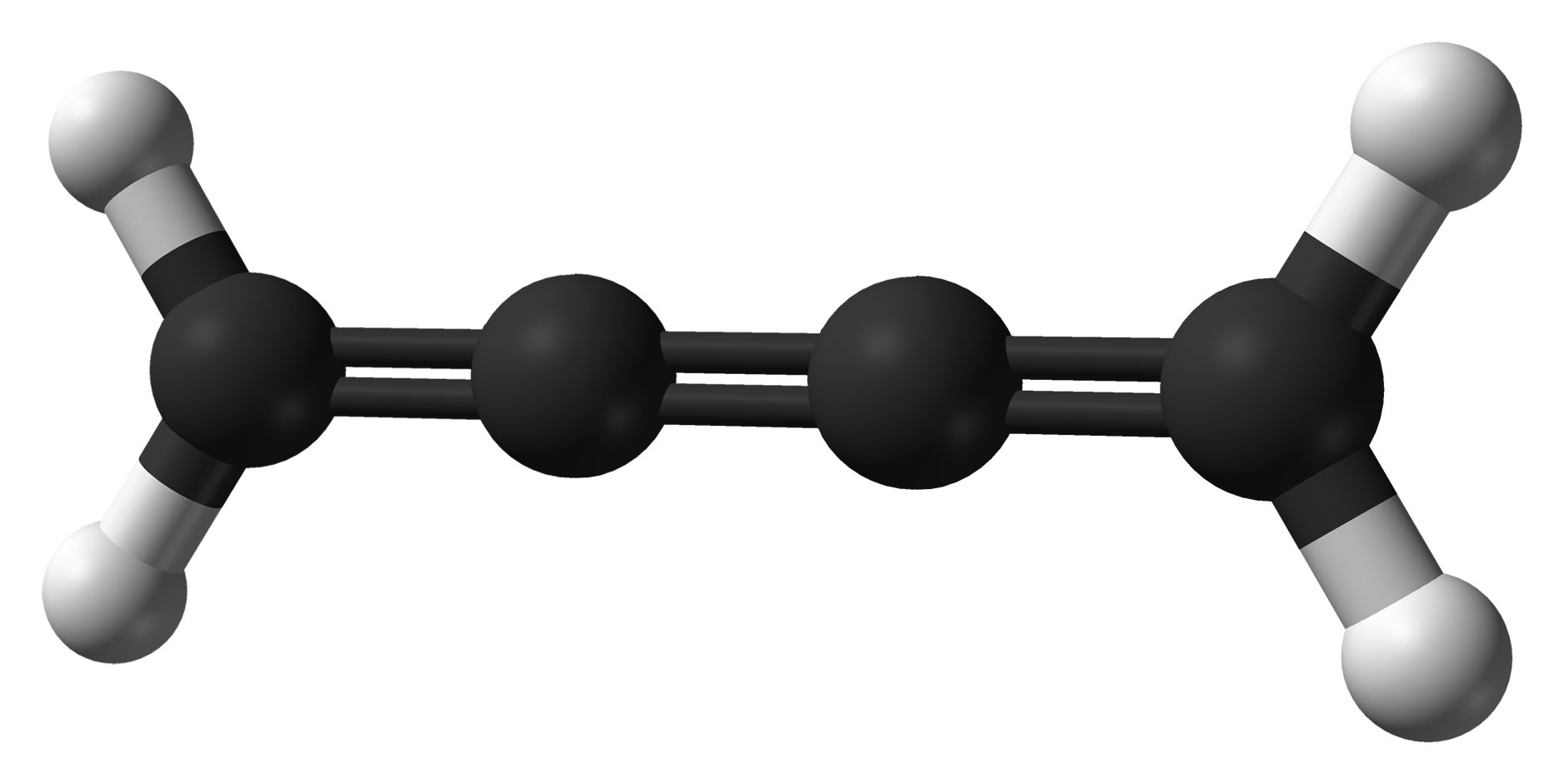
Diacetylene
Diacetylene (also known as butadiyne) is the organic compound with the formula or . It is the simplest compound containing two triple bonds. It is first in the series of polyynes, which are of theoretical but not of practical interest. Occurrence Diacetylene has been identified in the atmosphere of Titan and in the protoplanetary nebula CRL 618 by its characteristic vibrational spectrum. It is proposed to arise by a reaction between acetylene and the ethynyl radical (), which is produced when acetylene undergoes photolysis. This radical can in turn attack the triple bond in acetylene and react efficiently even at low temperatures. Diacetylene has also been detected on the Moon. Preparation This compound may be made by the dehydrohalogenation of 1,4-dichloro-2-butyne by potassium hydroxide (in alcoholic medium) at ~70°C: : The bis(trimethylsilyl)-protected derivative may be prepared by the Hay coupling of (trimethylsilyl)acetylene: : See also * Acetylene * Diiodobutadi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fritsch–Buttenberg–Wiechell Rearrangement
The Fritsch–Buttenberg–Wiechell rearrangement, named for Paul Ernst Moritz Fritsch (1859–1913), Wilhelm Paul Buttenberg, and Heinrich G. Wiechell, is a chemical reaction A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ... whereby a 1,1-diaryl-2-bromo-alkene rearrangement reaction, rearranges to a 1,2-diaryl-alkyne by reaction with a strong base such as an alkoxide. This rearrangement is also possible with alkyl substituents. Reaction mechanism The strong base deprotonates the vinylic hydrogen, which after alpha elimination forms a Vinyl group, vinyl carbene. A 1,2-aryl migration forms the 1,2-diaryl-alkyne product. The mechanism of the FBW rearrangement was a subject of on-surface studies where the vinyl radical was visualised with sub-atomic resolution. Scope One study ex ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diacetylene
Diacetylene (also known as butadiyne) is the organic compound with the formula or . It is the simplest compound containing two triple bonds. It is first in the series of polyynes, which are of theoretical but not of practical interest. Occurrence Diacetylene has been identified in the atmosphere of Titan and in the protoplanetary nebula CRL 618 by its characteristic vibrational spectrum. It is proposed to arise by a reaction between acetylene and the ethynyl radical (), which is produced when acetylene undergoes photolysis. This radical can in turn attack the triple bond in acetylene and react efficiently even at low temperatures. Diacetylene has also been detected on the Moon. Preparation This compound may be made by the dehydrohalogenation of 1,4-dichloro-2-butyne by potassium hydroxide (in alcoholic medium) at ~70°C: : The bis(trimethylsilyl)-protected derivative may be prepared by the Hay coupling of (trimethylsilyl)acetylene: : See also * Acetylene * Diiodobutadi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cumulene
A cumulene is a compound having three or more ''cumulative'' (consecutive) double bonds. They are analogous to allenes, only having a more extensive chain. The simplest molecule in this class is butatriene (), which is also called simply ''cumulene''. Unlike most alkanes and alkenes, cumulenes tend to be rigid, comparable to polyynes. Cumulene carbenes for ''n'' from 3 to 6 have been observed in interstellar molecular cloud A molecular cloud—sometimes called a stellar nursery if star formation is occurring within—is a type of interstellar cloud of which the density and size permit absorption nebulae, the formation of molecules (most commonly molecular hydrogen, ...s and in laboratory experiments by using microwave and infrared spectroscopy. (The more stable cumulenes are difficult to detect optically because they lack an electric dipole moment.) Cumulenes containing heteroatoms are called heterocumulenes; an example is carbon suboxide. Synthesis The first reporte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkyne
\ce \ce Acetylene \ce \ce \ce Propyne \ce \ce \ce \ce 1-Butyne In organic chemistry, an alkyne is an unsaturated hydrocarbon containing at least one carbon—carbon triple bond. The simplest acyclic alkynes with only one triple bond and no other functional groups form a homologous series with the general chemical formula . Alkynes are traditionally known as acetylenes, although the name ''acetylene'' also refers specifically to , known formally as ethyne using IUPAC nomenclature. Like other hydrocarbons, alkynes are generally hydrophobic. Structure and bonding In acetylene, the H–C≡C bond angles are 180°. By virtue of this bond angle, alkynes are rod-like. Correspondingly, cyclic alkynes are rare. Benzyne cannot be isolated. The C≡C bond distance of 118 picometers (for C2H2) is much shorter than the C=C distance in alkenes (132 pm, for C2H4) or the C–C bond in alkanes (153 pm). : The triple bond is very strong with a bond strength of 839 kJ/mol. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tris(3,5-di-t-butylphenyl)methyl
Tris, or tris(hydroxymethyl)aminomethane, or known during medical use as tromethamine or THAM, is an organic compound with the formula (HOCH2)3CNH2. It is extensively used in biochemistry and molecular biology as a component of buffer solutions such as in TAE and TBE buffers, especially for solutions of nucleic acids. It contains a primary amine and thus undergoes the reactions associated with typical amines, e.g., condensations with aldehydes. Tris also complexes with metal ions in solution. In medicine, tromethamine is occasionally used as a drug, given in intensive care for its properties as a buffer for the treatment of severe metabolic acidosis in specific circumstances. Some medications are formulated as the "tromethamine salt" including Hemabate (carboprost as trometamol salt), and "ketorolac trometamol". In 2023 a strain of ''Pseudomonas hunanensis'' was found to be able to degrade TRIS buffer. Since Tris' pKa is more strongly temperature dependent, its use is not recom ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen atom, which may be replaced by some other element or compound to serve as a functional group. A phenyl group has six carbon atoms bonded together in a hexagonal planar ring, five of which are bonded to individual hydrogen atoms, with the remaining carbon bonded to a substituent. Phenyl groups are commonplace in organic chemistry. Although often depicted with alternating double and single bonds, the phenyl group is chemically aromatic and has equal bond lengths between carbon atoms in the ring. Nomenclature Usually, a "phenyl group" is synonymous with and is represented by the symbol Ph (archaically, Φ), or Ø. Benzene is sometimes denoted as PhH. Phenyl groups are generally attached to other atoms or groups. Fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dendrimer
Dendrimers are highly ordered, Branching (polymer chemistry), branched molecules, polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The word dendron is also encountered frequently. A dendron usually contains a single chemically addressable group called the focal point or core. The difference between dendrons and dendrimers is illustrated in the top figure, but the terms are typically encountered interchangeably. The first dendrimers were made by #Divergent methods, divergent synthesis approaches by Fritz Vögtle in 1978, R.G. Denkewalter at Allied Corporation in 1981, Donald Tomalia at Dow Chemical in 1983 and in 1985, and by George R. Newkome in 1985. In 1990 a #Convergent methods, convergent synthetic approach was introduced by Craig Hawker and Jean Fréchet. Dendrimer popularity then greatly increased, resulting in more tha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |