|
Phenazine
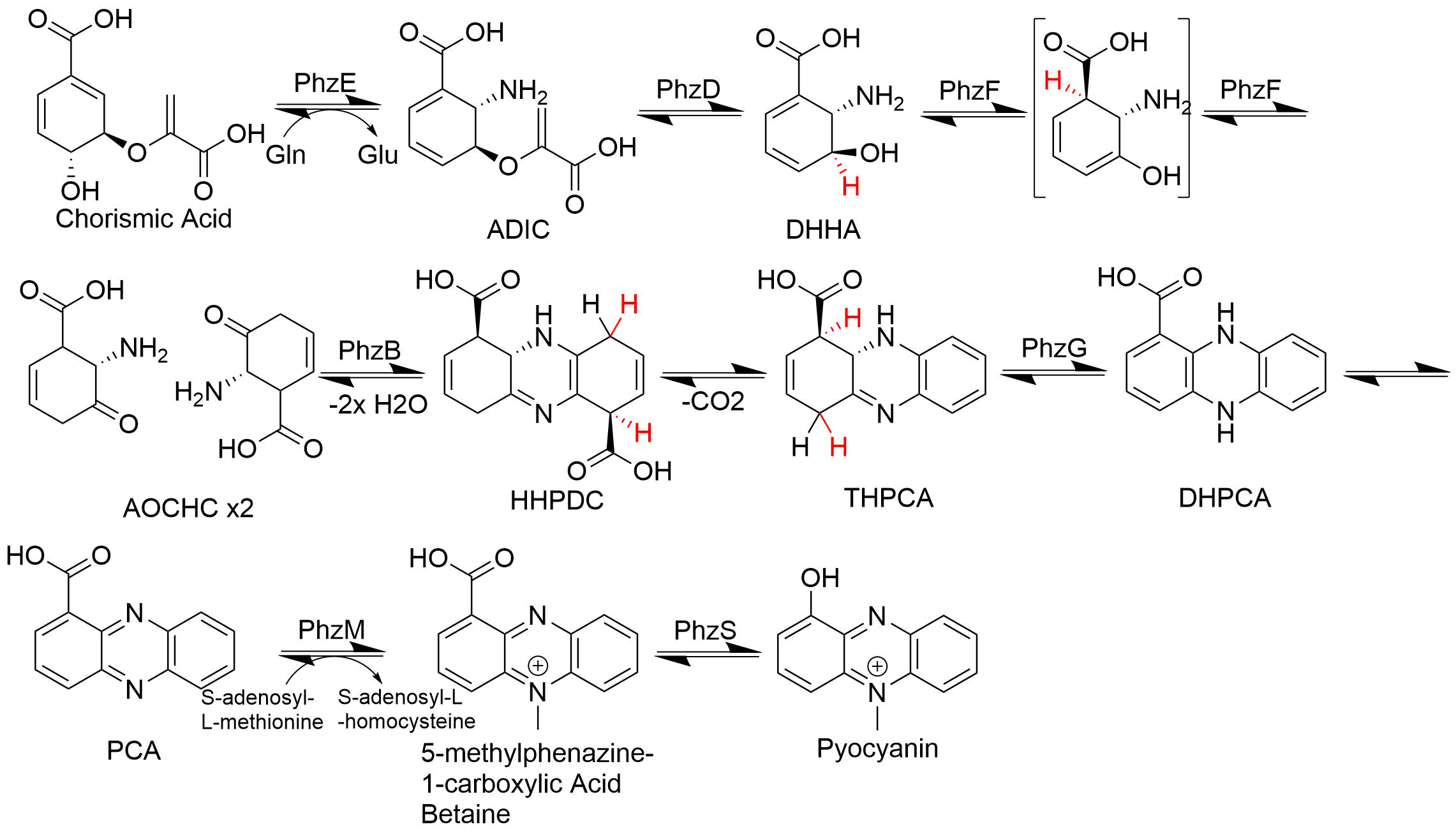
Phenazine is an organic compound with the formula (C6H4)2N2. It is a dibenzo annulation, annulated pyrazine, and the parent substance of many dyestuffs, such as the toluylene red, indulines, and safranines (and the closely related eurhodines). Phenazine crystallizes in yellow needles, which are only sparingly soluble in ethanol, alcohol. Sulfuric acid dissolves it, forming a deep-red solution. Synthesis Classically phenazine are prepared by the reaction of nitrobenzene and aniline in the Wohl–Aue reaction. Other methods include: * pyrolysis of the barium salt (chemistry), salt of azobenzoate * oxidation of aniline with lead oxide * oxidation of dihydrophenazine, which is prepared by heating pyrocatechin with o-phenylenediamine, ''o''-phenylenediamine. * oxidation of ortho-aminodiphenylamine with lead peroxide. Derivatives * The more complex phenazines, such as the naphthophenazines, naphthazines, and naphthotolazines, may be prepared by condensing Toluidine, ortho-diamines with ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Induline
Induline is a dye of blue, bluish-red or black shades. Induline consists of a mixture of several intensely colored species, so the name is often indulines. It was one of the first synthetic dyes, discovered in 1863 by J. Dale and Heinrich Caro. The main components of induline are various substituted phenazines. Although induline is no longer in use, the related dye nigrosin is still produced commercially. Relationship to other compounds Induline is a derivative of the eurhodines (aminophenazines, aminonaphthophenazines). By means of their diazo derivatives can be de-amidated, yielding in this way azonium salts; consequently they may be considered as amidated azonium salts. The first reaction giving a clue to their constitution was the isolation of the intermediate azophenin by O. Witt, which was proved by Fischer and Hepp to be dianilidoquinone dianil, a similar intermediate compound being found shortly afterwards in the naphthalene series. Azophenin, C30H24N4, is prepared by w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Toluylene Red
Neutral red (toluylene red, Basic Red 5, or C.I. 50040) is a eurhodin dye used for staining in histology. It stains lysosomes red. It is used as a general stain in histology, as a counterstain in combination with other dyes, and for many staining methods. Together with Janus Green B, it is used to stain embryonal tissues and supravital staining of blood. It can be used for staining the Golgi apparatus in cells and Nissl granules in neurons. In microbiology, it is used in the MacConkey agar to differentiate bacteria for lactose fermentation.Neutral redcan be used as a vital stain. The Neutral Red Cytotoxicity Assay was first developed by Ellen Borenfreund in 1984. In the Neutral Red Assay live cells incorporate neutral red into their lysosomes. As cells begin to die, their ability to incorporate neutral red diminishes. Thus, loss of neutral red uptake corresponds to loss of cell viability. The neutral red is also used to stain cell cultures for plate titration of viruses. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Safranine
Safranin (Safranin O or basic red 2) is a biological stain used in histology and cytology. Safranin is used as a counterstain in some staining protocols, colouring cell nuclei red. This is the classic counterstain in both Gram stains and endospore staining. It can also be used for the detection of cartilage, mucin and mast cell granules. Safranin typically has the chemical structure shown at right (sometimes described as dimethyl safranin). There is also trimethyl safranin, which has an added methyl group in the ''ortho-'' position (see Arene substitution pattern) of the lower ring. Both compounds behave essentially identically in biological staining applications, and most manufacturers of safranin do not distinguish between the two. Commercial safranin preparations often contain a blend of both types. Safranin is also used as redox indicator in analytical chemistry. Safranines Safranines are the azonium compounds of symmetrical 2,8-dimethyl-3,7-diaminophenazine. They ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Aniline
Aniline (From , meaning ' indigo shrub', and ''-ine'' indicating a derived substance) is an organic compound with the formula . Consisting of a phenyl group () attached to an amino group (), aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, aniline is "electron-rich". It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Ani ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemistry, chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 49,000 in the world. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham (chemist), Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, People' ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Toluidine
There are three isomers of toluidine, which are organic compounds discovered and named by James Sheridan Muspratt and August Wilhelm von Hofmann in 1845. These isomers are O-Toluidine, ''o''-toluidine, ''m''-toluidine, and ''p''-toluidine, with the prefixed letter abbreviating, respectively, arene substitution pattern#Ortho, meta, and para substitution, ''ortho''; ''meta''; and ''para''. All three are aryl amines whose chemical structures are similar to aniline except that a methyl group is substituted onto the benzene ring. The difference between these three isomers is the position where the methyl group (–CH3) is bonded to the ring relative to the amino functional group (–NH2); see illustration of the chemical structures below. The chemical properties of the toluidines are quite similar to those of aniline, and toluidines have properties in common with other aromatic amines. Due to the amino group bonded to the aromatic ring, the toluidines are weak base, weakly basic. The ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Quinone
The quinones are a class of organic compounds that are formally "derived from aromatic compounds benzene.html" ;"title="uch as benzene">uch as benzene or naphthalene] by conversion of an even number of –CH= groups into –C(=O)– groups with any necessary rearrangement of double bonds", resulting in "a fully Conjugated system, conjugated cyclic diketone, dione structure". The archetypical member of the class is 1,4-benzoquinone or cyclohexadienedione, often called simply "quinone" (thus the name of the class). Other important examples are 1,2-benzoquinone (''ortho''-quinone), 1,4-naphthoquinone and 9,10-anthraquinone. The name is derived from that of quinic acid (with the suffix "-one" indicating a ketone), since it is one of the compounds obtained upon oxidation of quinic acid. Quinic acid, like quinine is obtained from cinchona bark, called quinaquina in the indigenous languages of Peruvian tribes. Properties Quinones are oxidized derivatives of aromatic compounds an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acid–base theory, Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Acid–base reaction#Arrhenius theory, Arrhenius acids. Johannes Nicolaus Brønsted, Brønsted and Martin Lowry, Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
α-naphthol
1-Naphthol, or α-naphthol, is an organic compound with the formula . It is a fluorescence, fluorescent white solid. 1-Naphthol differs from its isomer 2-Naphthol, 2-naphthol by the location of the hydroxide, hydroxyl group on the naphthalene ring. The naphthols are naphthalene homologues of phenol. Both isomers are soluble in simple organic solvents. They are precursors to a variety of useful compounds. Production 1-Naphthol is prepared by two main routes. In one method, naphthalene is nitrated to give 1-nitronaphthalene, which is hydrogenated to the amine followed by hydrolysis: : : : Alternatively, naphthalene is hydrogenated to tetralin, which is oxidized to 1-tetralone, which undergoes dehydrogenation. Reactions Some reactions of 1-naphthol are explicable with reference to its tautomerism, which produces a small amount of the keto tautomer. : One consequence of this tautomerism is the Bucherer reaction, the ammonolysis of 1-naphthol to give 1-Aminonaphthylaminel, 1-ami ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Azo Compound
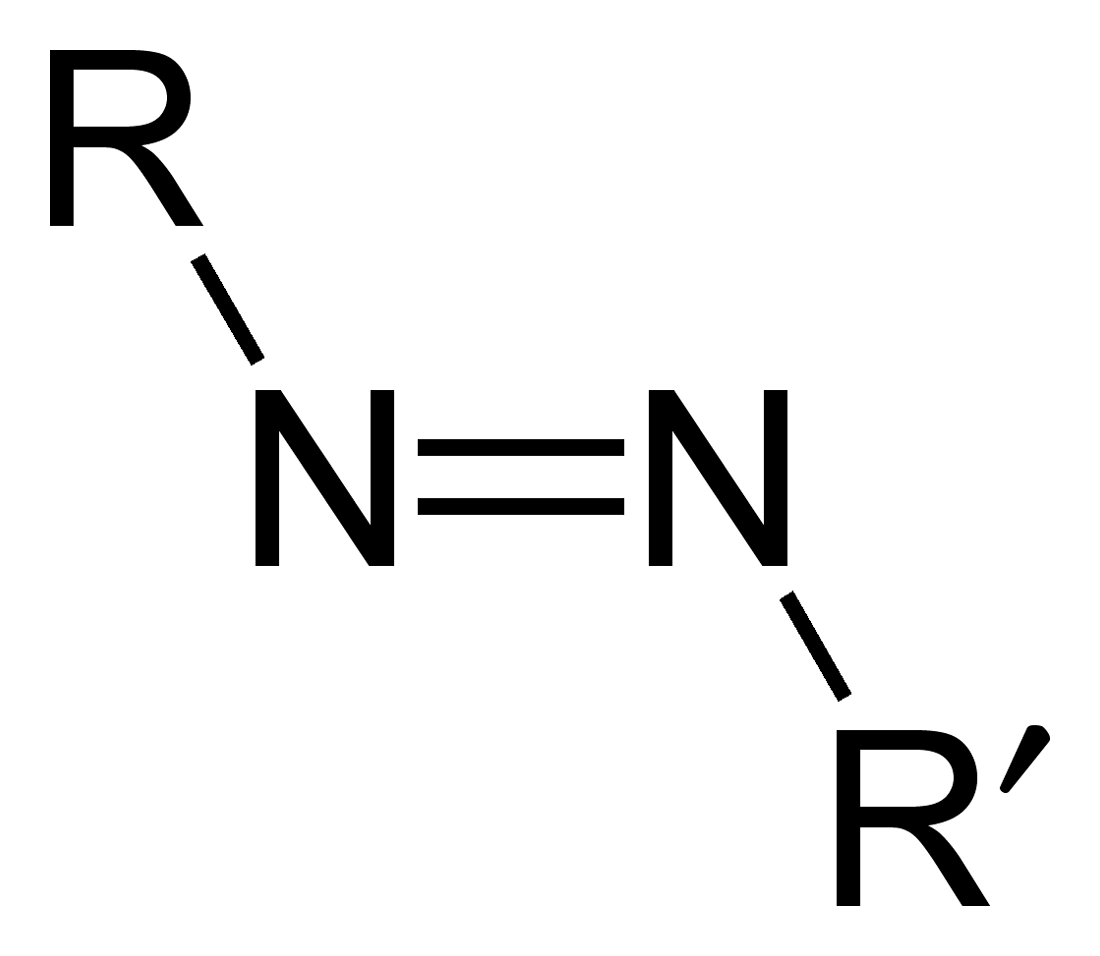
Azo compounds are organic compounds bearing the functional group diazenyl (, in which R and R′ can be either aryl or alkyl groups). IUPAC defines azo compounds as: "Derivatives of diazene (diimide), , wherein both hydrogens are substituted by hydrocarbyl groups, e.g. azobenzene or diphenyldiazene.", where Ph stands for phenyl group. The more stable derivatives contain two aryl groups. The group is called an ''azo group'' (, ). Many textile and leather articles are dyed with azo dyes and pigments. Aryl azo compounds urinary tract infections">Phenazopyridine, an aryl azo compound, is used to treat urinary tract infections">150px Aryl azo compounds are usually stable, crystalline species. Azobenzene is the prototypical aromatic azo compound. It exists mainly as the Cis-trans isomerism, ''trans'' isomer, but upon illumination, converts to the Cis-trans isomerism, ''cis'' isomer. Aromatic azo compounds can be synthesized by azo coupling, which entails an electrophilic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |