|
Perfluorooctane
Perfluorooctane, also known as octadecafluorooctane, is a fluorocarbon liquid—a perfluorinated derivative of the hydrocarbon octane. It can be a good substitute for insulating oil in high voltage electronics. In addition to heat transfer applications, it has also been used as a breathable fluid in partial liquid ventilation. Production Perfluorooctane can be produced by the Fowler process or by electrochemical fluorination. Fowler process The Fowler process involves moderating the action of elemental fluorine with cobalt fluoride in the gas phase from octane. Electrochemical fluorination Electrolysis in hydrogen fluoride of nonanoic acid will produce both perfluorononanoic acid and perfluorooctane. Perfluorooctane manufactured this way is marketed under the name PF5080 (or FC77) by 3M as part of their Fluorinert Fluorinert is the trademarked brand name for the line of electronics coolant liquids sold commercially by 3M. As perfluorinated compounds (PFCs), all Fluorinert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Liquid Breathing
Liquid breathing is a form of Respiration (physiology), respiration in which a normally Atmosphere of Earth, air-breathing organism breathes an oxygen-rich liquid which is capable of CO2 gas exchange (such as a perfluorocarbon). The liquid involved requires certain physical properties, such as respiratory gas solubility, density, viscosity, vapor pressure and lipid solubility, which some perfluorocarbon, perfluorochemicals (PFCs) have. Thus, it is critical to choose the appropriate PFC for a specific biomedical application, such as liquid ventilation, drug delivery or blood substitutes. The physical properties of PFC liquids vary substantially; however, the one common property is their high solubility for respiratory gases. In fact, these liquids carry more oxygen and carbon dioxide than blood. In theory, liquid breathing could assist in the treatment of patients with severe human lung, pulmonary or human heart, cardiac trauma, especially in pediatric cases. Liquid breathing h ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluorononanoic Acid
Perfluorononanoic acid, or PFNA, is a synthetic perfluorinated carboxylic acid and fluorosurfactant that is also a persistent organic pollutant. Chemistry and properties In acidic form it is a highly reactive strong acid. In its conjugate base form as a salt it is stable and commonly ion paired with ammonium. In the commercial product Surflon S-111 (CAS 72968-3-88) it is the primary compound present by weight. PFNA is used as surfactant for the production of the fluoropolymer polyvinylidene fluoride.Supporting Information (PDF) It is produced mainly in Japan by the of a linear |
Sigma-Aldrich
Sigma-Aldrich (formally MilliporeSigma) is an American chemical, life science, and biotechnology company owned by the multinational chemical conglomerate Merck Group. Sigma-Aldrich was created in 1975 by the merger of Sigma Chemical Company and Aldrich Chemical Company. It grew through various acquisitions until it had over 9,600 employees and was listed on the Fortune 1000. The company has two United States headquarters, in St. Louis and Burlington, MA and has operations in approximately 40 countries. In 2015, the multinational chemical conglomerate Merck Group acquired Sigma-Aldrich for $17 billion. The company is currently a part of Merck's life science business and in combination with Merck's earlier acquired Millipore Corporation, Millipore, operates as MilliporeSigma. It is headquartered in Burlington, Massachusetts, United States. History Sigma Chemical Company of St. Louis and Aldrich Chemical Company of Milwaukee were both American specialty chemical companies when they ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorocarbon
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics. Nomenclature Perfluorocarbons or PFCs, are organofluorine compounds with the formula CxFy, meaning they contain only carbon and fluorine. The terminology is not strictly followed and many fluorine-containing organic compounds are also called fluorocarbons. Compounds with the prefix perfluoro- are hydrocarbons, including those with heteroatoms, wherein all C-H bonds have been replaced by C-F bonds. Fluorocarbons includes perfluoroalkanes, fluoroalkenes, fluoroalkynes, and perfluoroaromatic compounds. Perfluoroalkanes Chemical properties Perfluoroalkanes are very stable because of the strength of the carbon–fluorine bond, one of the strongest in organic chemistry. Its st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually faint, and may be similar to that of gasoline or Naphtha, lighter fluid. They occur in a diverse range of molecular structures and phases: they can be gases (such as methane and propane), liquids (such as hexane and benzene), low melting solids (such as paraffin wax and naphthalene) or polymers (such as polyethylene and polystyrene). In the fossil fuel industries, ''hydrocarbon'' refers to naturally occurring petroleum, natural gas and coal, or their hydrocarbon derivatives and purified forms. Combustion of hydrocarbons is the main source of the world's energy. Petroleum is the dominant raw-material source for organic commodity chemicals such as solvents and polymers. Most anthropogenic (human-generated) emissions of greenhouse gases are eithe ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Octane
Octane is a hydrocarbon and also an alkane with the chemical formula C8H18, and the condensed structural formula CH3(CH2)6CH3. Octane has many structural isomers that differ by the location of branching in the carbon chain. One of these isomers, 2,2,4-Trimethylpentane, 2,2,4-trimethylpentane (commonly called iso-octane), is used as one of the standard values in the octane rating scale. Octane is a component of gasoline and petroleum. Under standard temperature and pressure, octane is an odorless, colorless liquid. Like other short-chained alkanes with a low molecular weight, it is Volatility (chemistry), volatile, flammable, and toxic. Octane is 1.2 to 2 times more toxic than heptane. Isomers N-octane has 23 Structural isomers, constitutional isomers. 8 of these isomers have one stereocenter; 3 of them have two stereocenters. Achiral isomers: * 2-Methylheptane * 4-Methylheptane * 3-Ethylhexane * 2,2-Dimethylhexane * 2,5-Dimethylhexane * 3,4-Dimethylhexane, (''meso'')-3,4-Dime ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fowler Process
The Fowler process is an industry and laboratory route to fluorocarbons, by fluorinating hydrocarbons or their partially fluorinated derivatives in the vapor phase over cobalt(III) fluoride. Background The Manhattan Project required the production and handling of uranium hexafluoride for uranium enrichment, whether by diffusion or centrifuge. Uranium hexafluoride is very corrosive, oxidising, volatile solid ( sublimes at 56 °C). To handle this material, several new materials were required, including a coolant liquid that could survive contact with uranium hexafluoride. Perfluorocarbons were identified as ideal materials, but at that point no method was available to produce them in any significant quantity. The problem is that fluorine gas is extremely reactive. Simply exposing a hydrocarbon to fluorine will cause the hydrocarbon to ignite. A way to moderate the reaction was required, and the method developed was to react the hydrocarbon with cobalt(III) fluoride, rather than ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochemical Fluorination
Electrochemical fluorination (ECF), or electrofluorination, is a foundational organofluorine chemistry method for the preparation of fluorocarbon-based organofluorine compounds.G. Siegemund, W. Schwertfeger, A. Feiring, B. Smart, F. Behr, H. Vogel, B. McKusick "Fluorine Compounds, Organic" in "Ullmann’s Encyclopedia of Industrial Chemistry" 2005, Wiley-VCH, Weinheim. The general approach represents an application of electrosynthesis. The fluorinated chemical compounds produced by ECF are useful because of their distinctive solvation properties and the relative inertness of carbon–fluorine bonds. Two ECF synthesis routes are commercialized and commonly applied: the Simons process and the Phillips Petroleum process. It is also possible to electrofluorinate in various organic media.Fred G. Drakesmith "Electrofluorination of Organic Compounds" Topics in Current Chemistry, Vol. 193, Springer, Berlin-Heidelberg, 1997. Prior to the development of these methods, fluorination with fluori ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
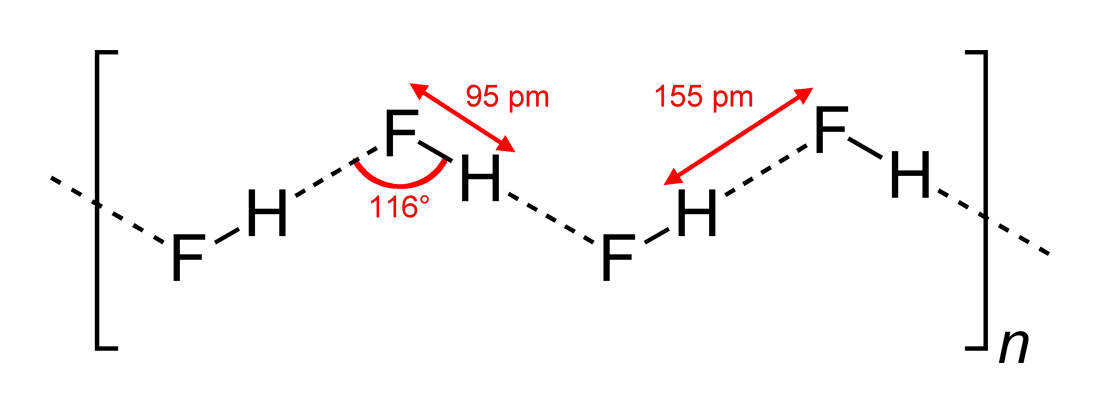
Hydrogen Fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals and polymers such as polytetrafluoroethylene (PTFE). HF is also widely used in the petrochemical industry as a component of superacids. Due to strong and extensive hydrogen bonding, it boils near room temperature, a much higher temperature than other hydrogen halides. Hydrogen fluoride is an extremely dangerous gas, forming corrosive and penetrating hydrofluoric acid upon contact with moisture. The gas can also cause blindness by rapid destruction of the corneas. History In 1771 Carl Wilhelm Scheele prepared the aqueous solution, hydrofluoric acid in large quantities, although hydrofluoric acid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nonanoic Acid
Pelargonic acid, also called nonanoic acid, is an organic compound with structural formula . It is a nine-carbon fatty acid. Nonanoic acid is a colorless oily liquid with an unpleasant, rancid odor. It is nearly insoluble in water, but very soluble in organic solvents. The esters and salts of pelargonic acid are called pelargonates or nonanoates. The acid is named after the pelargonium plant, since oil from its leaves contains esters of the acid. Preparation Together with azelaic acid, it is produced industrially by ozonolysis of oleic acid. : Alternatively, pelargonic acid can be produced in a two-step process beginning with coupled dimerization and hydroesterification of 1,3- butadiene. This step produces a doubly unsaturated C9-ester, which can be hydrogenated to give esters of pelargonic acid. : : A laboratory preparation involves permanganate oxidation of 1-decene. Occurrence and uses Pelargonic acid occurs naturally as esters in the oil of ''Pelargonium''. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fluorinert
Fluorinert is the trademarked brand name for the line of electronics coolant liquids sold commercially by 3M. As perfluorinated compounds (PFCs), all Fluorinert variants have an extremely high global warming potential (GWP), so should be used with caution (see below). It is an electrically insulating, stable fluorocarbon-based fluid, which is used in various cooling applications. It is mainly used for cooling electronics. Different molecular formulations are available with a variety of boiling points, allowing it to be used in "single-phase" applications, where it remains a liquid, or for "two-phase" applications, where the liquid boils to remove additional heat by evaporative cooling. An example of one of the compounds 3M uses is FC-72 ( perfluorohexane, C6F14). Perfluorohexane is used for low-temperature heat-transfer applications due to its boiling point. Another example is FC-75, perfluoro(2-butyl-tetrahydrofurane). There are 3M fluids that can handle up to , such as FC-70 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Perfluoroalkanes
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced stability, volatility, and hydrophobicity. Several fluorocarbons and their derivatives are commercial polymers, refrigerants, drugs, and anesthetics. Nomenclature Perfluorocarbons or PFCs, are organofluorine compounds with the formula CxFy, meaning they contain only carbon and fluorine. The terminology is not strictly followed and many fluorine-containing organic compounds are also called fluorocarbons. Compounds with the prefix perfluoro- are hydrocarbons, including those with heteroatoms, wherein all C-H bonds have been replaced by C-F bonds. Fluorocarbons includes perfluoroalkanes, fluoroalkenes, fluoroalkynes, and perfluoroaromatic compounds. Perfluoroalkanes Chemical properties Perfluoroalkanes are very stable because of the strength of the carbon–fluorine bond, one of the strongest in organic chemistry. Its stre ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |