|
Percent Active Chlorine
Percent active chlorine is a unit of concentration used for hypochlorite-based bleaches. One gram of a 100% active chlorine bleach has the quantitative bleaching capacity as one gram of free chlorine. The term "active chlorine" is used because most commercial bleaches also contain chlorine in the form of chloride ions, which have no bleaching properties. Liquid bleaches for domestic use fall in 3 categories: for pool-treatment (10% hypochlorite solutions, without surfactants and detergents), for laundry and general purpose cleaning, at 3–5% active chlorine (which are usually recommended to be diluted substantially before use), and in pre-mixed specialty formulations targeted at particular cleaning, bleaching or disinfecting applications. Commercial chlorine bleaches range from under 10% active chlorine to over 40%. Values can be higher than 100% because hypochlorite ion has a molecular weight of 51.45 g/mol, whereas dichlorine Cl2 has a molecular weight of 70.90 g/mol. D ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Concentration
In chemistry, concentration is the abundance of a constituent divided by the total volume of a mixture. Several types of mathematical description can be distinguished: '' mass concentration'', '' molar concentration'', '' number concentration'', and '' volume concentration''. The concentration can refer to any kind of chemical mixture, but most frequently refers to solutes and solvents in solutions. The molar (amount) concentration has variants, such as normal concentration and osmotic concentration. Dilution is reduction of concentration, e.g. by adding solvent to a solution. The verb to concentrate means to increase concentration, the opposite of dilute. Etymology ''Concentration-'', ''concentratio'', action or an act of coming together at a single place, bringing to a common center, was used in post-classical Latin in 1550 or earlier, similar terms attested in Italian (1589), Spanish (1589), English (1606), French (1632). Qualitative description Often in informal, non- ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Calcium Hypochlorite
Calcium hypochlorite is an inorganic compound with chemical formula , also written as . It is a white solid, although commercial samples appear yellow. It strongly smells of chlorine, owing to its slow decomposition in moist air. This compound is relatively stable as a solid and solution and has greater available chlorine than sodium hypochlorite. "Pure" samples have 99.2% active chlorine. Given common industrial purity, an active chlorine content of 65-70% is typical. It is the main active ingredient of commercial products called bleaching powder, used for water treatment and as a bleaching agent. History Charles Tennant and Charles Macintosh developed an industrial process in the late 18th century for the manufacture of chloride of lime, patenting it in 1799. Tennant's process is essentially still used today, and became of military importance during World War I, because calcium hypochlorite was the active ingredient in trench disinfectant. Uses Sanitation Calcium hypochlor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
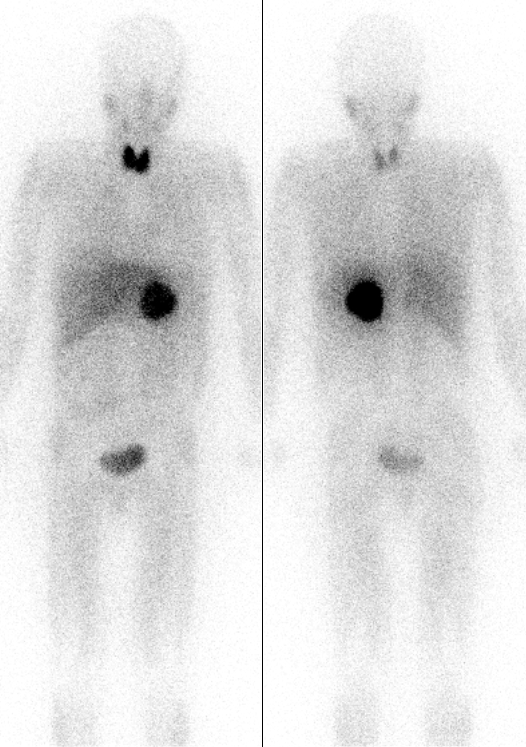
Iodine
Iodine is a chemical element; it has symbol I and atomic number 53. The heaviest of the stable halogens, it exists at standard conditions as a semi-lustrous, non-metallic solid that melts to form a deep violet liquid at , and boils to a violet gas at . The element was discovered by the French chemist Bernard Courtois in 1811 and was named two years later by Joseph Louis Gay-Lussac, after the Ancient Greek , meaning 'violet'. Iodine occurs in many oxidation states, including iodide (I−), iodate (), and the various periodate anions. As the heaviest essential mineral nutrient, iodine is required for the synthesis of thyroid hormones. Iodine deficiency affects about two billion people and is the leading preventable cause of intellectual disabilities. The dominant producers of iodine today are Chile and Japan. Due to its high atomic number and ease of attachment to organic compounds, it has also found favour as a non-toxic radiocontrast material. Because of the spec ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Titration
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of Quantitative research, quantitative Analytical chemistry, chemical analysis to determine the concentration of an identified analyte (a substance to be analyzed). A reagent, termed the ''titrant'' or ''titrator'', is prepared as a standard solution of known concentration and volume. The titrant reacts with a Solution (chemistry), solution of ''analyte'' (which may also be termed the ''titrand'') to determine the analyte's concentration. The volume of titrant that reacted with the analyte is termed the ''titration volume''. History and etymology The word "titration" descends from the French word ''titrer'' (1543), meaning the proportion of gold or silver in coins or in works of gold or silver; i.e., a measure of fineness or purity. ''Tiltre'' became ''titre'', which thus came to mean the "fineness of alloyed gold", and then the "concentration of a substance in a given sample". In 1828, t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Potassium Iodide
Potassium iodide is a chemical compound, medication, and dietary supplement. It is a medication used for treating hyperthyroidism, in radiation emergencies, and for protecting the thyroid gland when certain types of radiopharmaceuticals are used. It is also used for treating skin sporotrichosis and phycomycosis. It is a supplement used by people with low dietary intake of iodine. It is administered orally. Common side effects include vomiting, diarrhea, abdominal pain, rash, and swelling of the salivary glands. Other side effects include allergic reactions, headache, goitre, and depression. While use during pregnancy may harm the baby, its use is still recommended in radiation emergencies. Potassium iodide has the chemical formula K I. Commercially it is made by mixing potassium hydroxide with iodine. Potassium iodide has been used medically since at least 1820. It is on the World Health Organization's List of Essential Medicines. Potassium iodide is available as a g ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Molar Mass
In chemistry, the molar mass () (sometimes called molecular weight or formula weight, but see related quantities for usage) of a chemical substance ( element or compound) is defined as the ratio between the mass () and the amount of substance (, measured in moles) of any sample of the substance: . The molar mass is a bulk, not molecular, property of a substance. The molar mass is a ''weighted'' ''average'' of many instances of the element or compound, which often vary in mass due to the presence of isotopes. Most commonly, the molar mass is computed from the standard atomic weights and is thus a terrestrial average and a function of the relative abundance of the isotopes of the constituent atoms on Earth. The molecular mass (for molecular compounds) and formula mass (for non-molecular compounds, such as ionic salts) are commonly used as synonyms of molar mass, as the numerical values are identical (for all practical purposes), differing only in units ( dalton vs. g/mol o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Hypochlorite
In chemistry, hypochlorite, or chloroxide is an oxyanion with the chemical formula ClO−. It combines with a number of cations to form hypochlorite salts. Common examples include sodium hypochlorite (household bleach) and calcium hypochlorite (a component of bleaching powder, swimming pool "chlorine"). The Cl–O distance in ClO− is 1.69 Å. The name can also refer to esters of hypochlorous acid, namely organic compounds with a ClO– group covalently bound to the rest of the molecule. The principal example is ''tert''-butyl hypochlorite, which is a useful chlorinating agent. Most hypochlorite salts are handled as aqueous solutions. Their primary applications are as bleaching, disinfection, and water treatment agents. They are also used in chemistry for chlorination and oxidation reactions. Reactions Acid reaction Acidification of hypochlorites generates hypochlorous acid, which exists in an equilibrium with chlorine. A lowered pH (i.e. towards acid) drives the fo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |