|
Para-ethoxyamphetamine
''para''-Ethoxyamphetamine, also known as 4-ethoxyamphetamine (4-ETA), is a psychoactive drug and research chemical of the phenethylamine and amphetamine chemical classes which is closely related to the infamous ''para''-methoxyamphetamine (PMA). ''para''-Ethoxyamphetamine has similar effects to PMA in animal studies, although with slightly weaker stimulant effects. Like PMA, it has prominent MAOI activity, and is likely to have similar dangers associated with its use. See also * Substituted methoxyphenethylamine * ''para''-Methoxyamphetamine (PMA) * 2,5-Dimethoxy-4-ethoxyamphetamine (MEM) * 2,5-Dimethoxy-4-propoxyamphetamine MPM, also known as 2,5-dimethoxy-4-propoxyamphetamine, is a lesser-known putative psychedelic drug of the phenethylamine, amphetamine, and DOx families. It is a derivative of the DOx psychedelics TMA-2 and MEM in which the 4-position substituent ... (MPM) References {{DEFAULTSORT:Ethoxyamphetamine, 4- Methoxyphenethylamines Substituted ampheta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Substituted Methoxyphenethylamine
Methoxyphenethylamines (MPEAs), as well as methoxyamphetamines (MAs) in the case of the amphetamine (α-methylphenethylamine) homologues, are substituted phenethylamines with one or more methoxy groups. In some cases, one or more of the methoxy groups may also be extended to form other alkoxy and related groups such as ethoxy or propoxy. Methoxyphenethylamines may have additional substitutions as well. Many methoxyphenethylamines that have multiple methoxy groups in the 2- through 5-positions of the phenyl ring, for instance mescaline, 2C-B, TMA, DOM, and 25I-NBOMe, are serotonin 5-HT2A receptor agonists and serotonergic psychedelics. Other methoxyphenethylamines, particularly monomethoxyamphetamines like ''para''-methoxyamphetamine (PMA), are monoamine releasing agents of serotonin, norepinephrine, and/or dopamine, with stimulant and/or entactogen-related effects. Compounds closely related to methoxyphenethylamines include methylenedioxyphenethylamines (MDxx) like M ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Schedule I Drugs (US)
This is the list of Schedule I controlled substances in the United States as defined by the Controlled Substances Act.21 CFRbr>1308.11 (CSA Sched I) with changes through (Oct 18, 2012). Retrieved September 6, 2013. The following findings are required for substances to be placed in this schedule: United States Code via Cornell University's Legal Information Institute The Legal Information Institute (LII) is a non-profit public service of Cornell Law School that provides no-cost access to current American and international legal research sources online. Founded in 1992 by Peter Martin and Tom Bruce, LII .... Retrieved on 2007-10-02. # The drug or other substance has a high potential for abuse. # The drug or other substance has no currently accepted medical use in treatment in the United States. # There is a lack of accepted safety for use of the drug or other substance under medical supervision. The complete list of Schedule I substances is as follows. The Administrative C ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psychoactive Drug
A psychoactive drug, psychopharmaceutical, mind-altering drug, consciousness-altering drug, psychoactive substance, or psychotropic substance is a chemical substance that alters psychological functioning by modulating central nervous system activity. Psychoactive and psychotropic drugs both affect the brain, with psychotropics sometimes referring to psychiatric drugs or high-abuse substances, while “drug” can have negative connotations. Designer drug, Novel psychoactive substances are designer drugs made to mimic illegal ones and bypass laws. Psychoactive drug use dates back to prehistory for medicinal and consciousness-altering purposes, with evidence of widespread cultural use. Many animals intentionally consume psychoactive substances, and some traditional legends suggest animals first introduced humans to their use. Psychoactive substances are used across cultures for purposes ranging from medicinal and therapeutic treatment of Mental disorder, mental disorders and pain, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Research Chemical
Research chemicals are chemical substances which scientists use for medical and scientific research purposes. One characteristic of a research chemical is that it is for laboratory research use only; a research chemical is not intended for human or veterinary use. In the United States, this distinction is required on the labels of research chemicals and exempts them from regulation under parts 100-740 in Title 21 of the Code of Federal Regulations ( 21CFR). Background Agricultural research chemicals Research agrochemicals are created and evaluated to select effective substances for commercial off-the-shelf end-user products. Many research agrochemicals are never publicly marketed. Agricultural research chemicals often use sequential code name A code name, codename, call sign, or cryptonym is a code word or name used, sometimes clandestinely, to refer to another name, word, project, or person. Code names are often used for military purposes, or in espionage. They may also be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenethylamine
Phenethylamine (PEA) is an organic compound, natural monoamine alkaloid, and trace amine, which acts as a central nervous system stimulant in humans. In the brain, phenethylamine regulates monoamine neurotransmission by binding to trace amine-associated receptor 1 (TAAR1) and inhibiting vesicular monoamine transporter 2 (VMAT2) in monoamine neurons. To a lesser extent, it also acts as a neurotransmitter in the human central nervous system. In mammals, phenethylamine is produced from the amino acid L-phenylalanine by the enzyme aromatic L-amino acid decarboxylase via enzymatic decarboxylation. In addition to its presence in mammals, phenethylamine is found in many other organisms and foods, such as chocolate, especially after microbial fermentation. Phenethylamine is sold as a dietary supplement for purported mood and weight loss-related therapeutic benefits; however, in orally ingested phenethylamine, a significant amount is metabolized in the small intestine by mon ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Amphetamine
Amphetamine (contracted from Alpha and beta carbon, alpha-methylphenethylamine, methylphenethylamine) is a central nervous system (CNS) stimulant that is used in the treatment of attention deficit hyperactivity disorder (ADHD), narcolepsy, and obesity; it is also used to treat binge eating disorder in the form of its inactive prodrug lisdexamfetamine. Amphetamine was discovered as a chemical in 1887 by Lazăr Edeleanu, and then as a drug in the late 1920s. It exists as two enantiomers: levoamphetamine and dextroamphetamine. ''Amphetamine'' properly refers to a specific chemical, the Racemic mixture, racemic free base, which is equal parts of the two enantiomers in their pure amine forms. The term is frequently used informally to refer to any combination of the enantiomers, or to either of them alone. Historically, it has been used to treat nasal congestion and depression. Amphetamine is also used as an Performance-enhancing substance, athletic performance enhancer and Nootropic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Class
Chemical classification systems attempt to classify elements or compounds according to certain chemical functional or structural properties. Whereas the structural properties are largely intrinsic, functional properties and the derived classifications depend to a certain degree on the type of chemical interaction partners on which the function is exerted. Sometimes other criteria like purely physical ones (e.g. molecular weight) or – on the other hand – functional properties above the chemical level are also used for building chemical taxonomies. Some systems mix the various levels, resulting in hierarchies where the domains are slightly confused, for example having structural and functional aspects end up on the same level. Whereas chemical function is closely dependent on chemical structure, the situation becomes more involved when e.g. pharmacological function is integrated, because the QSAR can usually not be directly computed from structural qualities. Physico-ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Para-Methoxyamphetamine
''para''-Methoxyamphetamine (PMA), also known as 4-methoxyamphetamine (4-MA), is a designer drug of the amphetamine class with serotonergic effects. Unlike other similar drugs of this family, PMA does not produce stimulant, euphoriant, or entactogen effects, and behaves more like an antidepressant in comparison, though it does have some psychedelic properties. PMA has been found in tablets touted as MDMA (ecstasy) although its effects are markedly different compared to those of MDMA. The consequences of such deception have often included hospitalization and death for unwitting users. PMA is commonly synthesized from anethole, the flavor compound of anise and fennel, mainly because the starting material for MDMA, safrole, has become less available due to law enforcement action, causing illicit drug manufacturers to use anethole as an alternative. Effects According to Alexander Shulgin in ''PiHKAL'', the effects of PMA at doses of 50 to 80mg included hypertension, diethyltry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
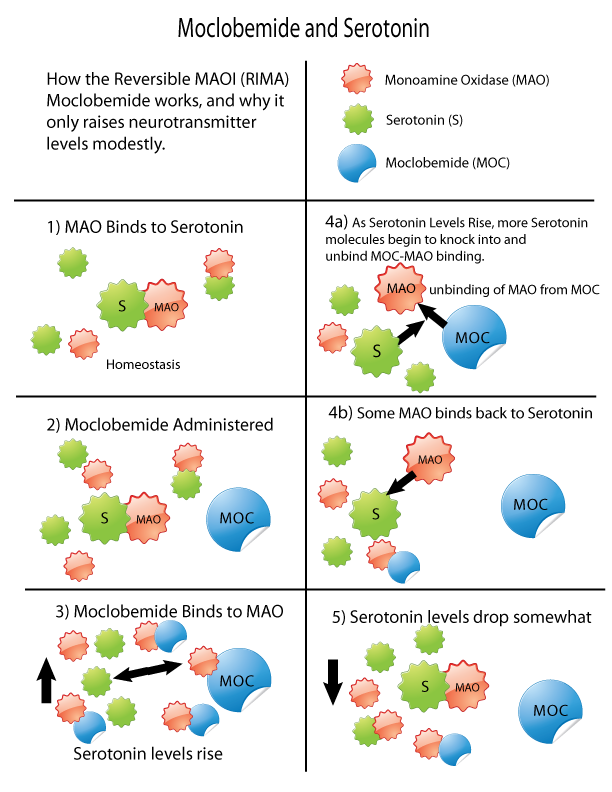
Monoamine Oxidase Inhibitor
Monoamine oxidase inhibitors (MAOIs) are a drug class, class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressants, especially for treatment-resistant depression and atypical depression. They are also used to treat panic disorder, social anxiety disorder, Parkinson's disease, and several other disorders. Reversible inhibitors of monoamine oxidase A (RIMAs) are a subclass of MAOIs that binding selectivity, selectively and Enzyme inhibitor#Reversible inhibitors, reversibly enzyme inhibitor, inhibit the MAO-A enzyme. RIMAs are used clinically in the medication, treatment of major depressive disorder, depression and dysthymia. Due to their reversibility, they are safer in single-drug overdose than the older, irreversible MAOIs, and weaker in increasing the monoamines important in depressive disorder. RIMAs have not gained widespread market share in th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Para-Methoxyamphetamine
''para''-Methoxyamphetamine (PMA), also known as 4-methoxyamphetamine (4-MA), is a designer drug of the amphetamine class with serotonergic effects. Unlike other similar drugs of this family, PMA does not produce stimulant, euphoriant, or entactogen effects, and behaves more like an antidepressant in comparison, though it does have some psychedelic properties. PMA has been found in tablets touted as MDMA (ecstasy) although its effects are markedly different compared to those of MDMA. The consequences of such deception have often included hospitalization and death for unwitting users. PMA is commonly synthesized from anethole, the flavor compound of anise and fennel, mainly because the starting material for MDMA, safrole, has become less available due to law enforcement action, causing illicit drug manufacturers to use anethole as an alternative. Effects According to Alexander Shulgin in ''PiHKAL'', the effects of PMA at doses of 50 to 80mg included hypertension, diethyltry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,5-Dimethoxy-4-ethoxyamphetamine
2,5-Dimethoxy-4-ethoxyamphetamine (MEM) is a psychedelic drug of the phenethylamine, amphetamine, and DOx families. It was first described by Alexander Shulgin by 1968. Dosage and effects In his book ''PiHKAL'', Alexander Shulgin lists the active dose range of MEM as 20 to 50mg orally and the duration as 10 to 14hours. According to Shulgin, MEM produces color enhancement, visual phenomena, and pattern movement, among other effects. Pharmacology MEM is a serotonergic psychedelic and acts as a selective serotonin 5-HT2 receptor agonist. It is specifically a full agonist of the serotonin 5-HT2A and 5-HT2C receptors and to a lesser extent is a partial to full agonist of the serotonin 5-HT2B receptor. The psychedelic effects of MEM are thought to be mediated by serotonin 5-HT2A receptor activation. Chemistry MEM, also known as 2,5-dimethoxy-4-ethoxyamphetamine, is a phenethylamine, amphetamine, and DOx derivative. It is the analogue and derivative of 2,4,5-trimethoxyam ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,5-Dimethoxy-4-propoxyamphetamine
MPM, also known as 2,5-dimethoxy-4-propoxyamphetamine, is a lesser-known putative psychedelic drug of the phenethylamine, amphetamine, and DOx families. It is a derivative of the DOx psychedelics TMA-2 and MEM in which the 4-position substituent has been extended. The drug is also the α-methyl or amphetamine analogue of 2C-O-7. Use and effects In his book ''PiHKAL'' (''Phenethylamines I Have Known and Loved''), Alexander Shulgin gives the dose range as "30mg or more" and the duration as "probably short". The drug produced weak or threshold effects at doses of 15 to 30mg. In another publication, Shulgin estimated an effective dose of MPM to be around 50mg and the drug as being around half as potent as TMA-2 or MEM. Pharmacology MPM produces the head-twitch response, a behavioral proxy of psychedelic effects, in rodents. It shows about the same potency as TMA-2 and MEM in this test. History MPM was first described in the scientific literature by Shulgin in 1978. Subsequently, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |