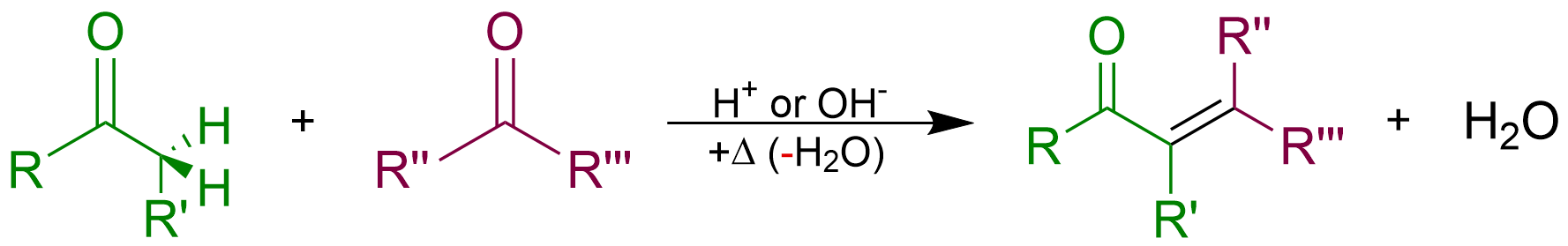|
Paclobutrazol
Paclobutrazol (PBZ) is the ISO common name for an organic compound that is used as a plant growth retardant and triazole fungicide. It is a known antagonist of the plant hormone gibberellin, acting by inhibiting gibberellin biosynthesis, reducing internodal growth to give stouter stems, increasing root growth, causing early fruitset and increasing seedset in plants such as tomato and pepper. PBZ has also been shown to reduce frost sensitivity in plants. Moreover, paclobutrazol can be used as a chemical approach for reducing the risk of lodging in cereal crops. PBZ has been used by arborists to reduce shoot growth and shown to have additional positive effects on trees and shrubs. Among those are improved resistance to drought stress, darker green leaves, higher resistance against fungi and bacteria, and enhanced development of roots. Cambial growth, as well as shoot growth, has been shown to be reduced in some tree species. Structure and synthesis The first synthesis of pacl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
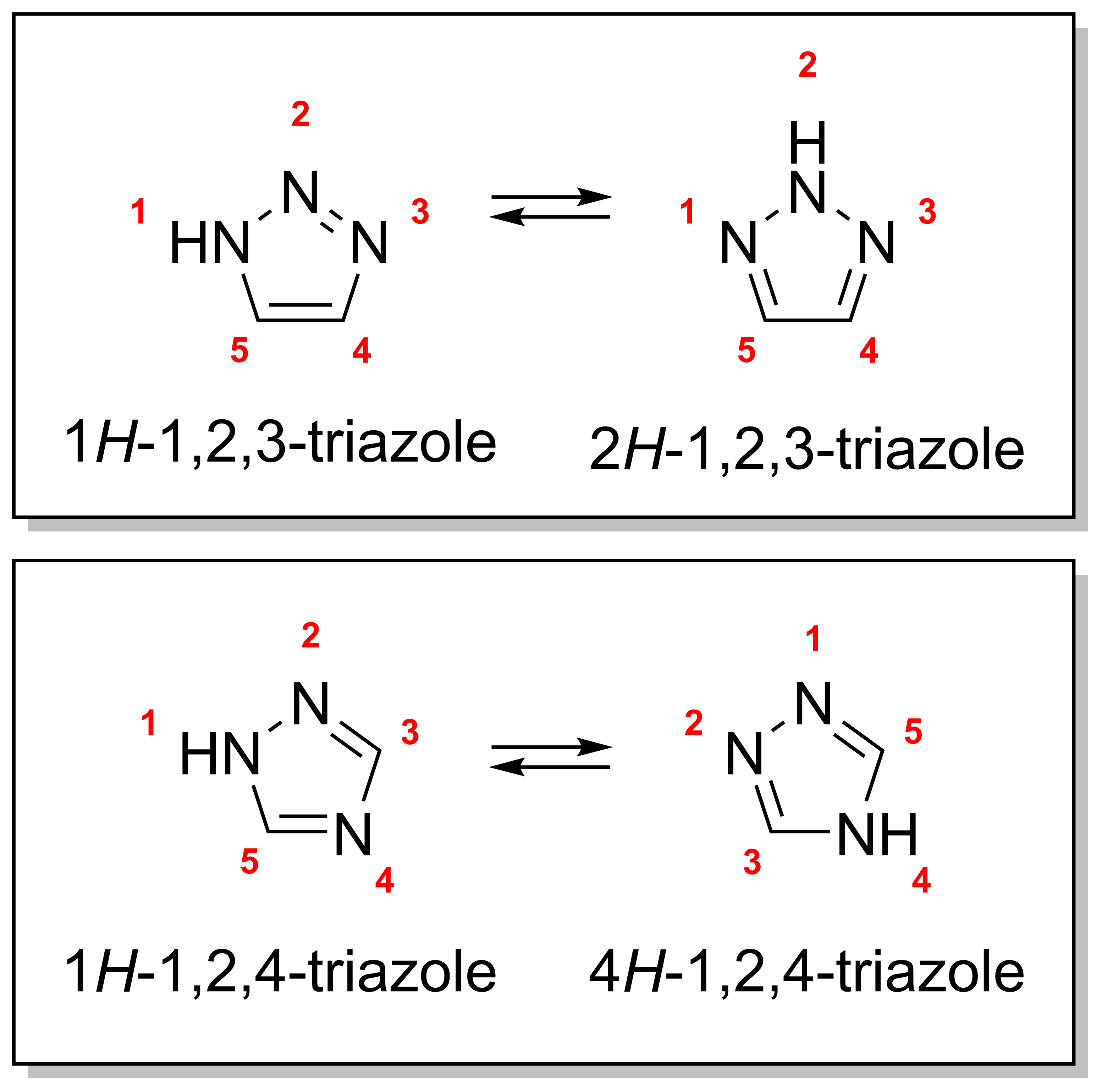
Triazole
A triazole is a heterocyclic compound featuring a five-membered ring of two carbon atoms and three nitrogen atoms with molecular formula C2H3N3. Triazoles exhibit substantial isomerism, depending on the positioning of the nitrogen atoms within the ring. Many triazoles are versatile, biologically active compounds commonly used as fungicides and plant retardants. However, triazoles are also useful in bioorthogonal chemistry, because the large number of nitrogen atoms causes triazoles to react similar to azides. Lastly, the many free lone pairs in triazoles make them useful as coordination compounds, although not typically as haptic ligands. Isomerism There are four triazole isomers, which are conventionally divided into two pairs of tautomers. In the 1,2,3-triazoles, the three nitrogen atoms are adjacent; in the 1,2,4-triazoles, an interstitial carbon separates out one nitrogen atom. Each category has two tautomers that differ by which nitrogen has a hydrogen bond ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gibberellin
Gibberellins (GAs) are plant hormones that regulate various developmental processes, including stem elongation, germination, dormancy, flowering, flower development, and leaf and fruit senescence. GAs are one of the longest-known classes of plant hormone. It is thought that the selective breeding (albeit unconscious) of crop strains that were deficient in GA synthesis was one of the key drivers of the " green revolution" in the 1960s, a revolution that is credited to have saved over a billion lives worldwide. History The first inroads into the understanding of GAs were developments from the plant pathology field, with studies on the '' bakanae'', or "foolish seedling" disease in rice. Foolish seedling disease causes a strong elongation of rice stems and leaves and eventually causes them to topple over. In 1926, Japanese scientist Eiichi Kurosawa identified that foolish seedling disease was caused by the fungus ''Gibberella fujikuroi.'' Later work at the University of Tokyo show ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International Organization For Standardization
The International Organization for Standardization (ISO ) is an international standard development organization composed of representatives from the national standards organizations of member countries. Membership requirements are given in Article 3 of the ISO Statutes. ISO was founded on 23 February 1947, and (as of November 2022) it has published over 24,500 international standards covering almost all aspects of technology and manufacturing. It has 809 Technical committees and sub committees to take care of standards development. The organization develops and publishes standardization in all technical and nontechnical fields other than electrical and electronic engineering, which is handled by the IEC.Editors of Encyclopedia Britannica. 3 June 2021.International Organization for Standardization" ''Encyclopedia Britannica''. Retrieved 2022-04-26. It is headquartered in Geneva, Switzerland, and works in 167 countries . The three official languages of the ISO are English, F ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tree
In botany, a tree is a perennial plant with an elongated stem, or trunk, usually supporting branches and leaves. In some usages, the definition of a tree may be narrower, including only woody plants with secondary growth, plants that are usable as lumber or plants above a specified height. In wider definitions, the taller palms, tree ferns, bananas, and bamboos are also trees. Trees are not a taxonomic group but include a variety of plant species that have independently evolved a trunk and branches as a way to tower above other plants to compete for sunlight. The majority of tree species are angiosperms or hardwoods; of the rest, many are gymnosperms or softwoods. Trees tend to be long-lived, some reaching several thousand years old. Trees have been in existence for 370 million years. It is estimated that there are some three trillion mature trees in the world. A tree typically has many secondary branches supported clear of the ground by the trunk. This trunk typi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to give a conjugated enone. The overall reaction is as follows (where the Rs can be H): Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or "aldol" (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. The term ''aldol condensation'' is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, this is formally an addition reaction rather than a condensation reaction because it does n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pinacolone
Pinacolone (3,3-dimethyl-2-butanone) is an important ketone in organic chemistry. It is a colorless liquid and has a slight peppermint- or camphor- odor. It is a precursor to triazolylpinacolone in the synthesis of the fungicide triadimefon and in synthesis of the herbicide metribuzin. The molecule is an unsymmetrical ketone. The α-methyl group can participate in condensation reactions. The carbonyl group can undergo the usual reactions (hydrogenation, reductive amination, etc.). It is a Schedule 3 compound under the Chemical Weapons Convention 1993, due to being related to pinacolyl alcohol, which is used in the production of soman. It is also a controlled export in Australia Group member states. Preparation Most famously, at least in the classroom, pinacolone arises by the pinacol rearrangement, which occurs by protonation of pinacol (2,3-dimethylbutane-2,3-diol). : Industrially pinacolone is made by the hydrolysis of 4,4,5-trimethyl-1,3-dioxane, which is the product of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Chlorobenzaldehyde
4-Chlorobenzaldehyde is an organic compound with the chemical formula C7H5ClO. It can produced by the oxidation of 4-chlorobenzyl alcohol. It can be further oxidized to 4-chlorobenzoic acid. It will react with malononitrile to form 4-chlorobenzylidenylmalononitrile. 4-Chlorobenzaldehyde reacts with benzylamine Benzylamine is an organic chemical compound with the condensed structural formula C6H5CH2NH2 (sometimes abbreviated as PhCH2NH2 or BnNH2). It consists of a benzyl group, C6H5CH2, attached to an amine functional group, NH2. This colorless wate ... to produce ''N''-(4-chlorobenzylidenyl)benzylamine。 References {{DEFAULTSORT:Chlorobenzaldehyde, 4- Chlorobenzenes Benzaldehydes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Jealott's Hill
Jealott's Hill is a village in the county of Berkshire, England, within the civil parish of Warfield. The settlement is on the A3095 road approximately north of Bracknell. The nearest railway station is in . The name of the hill is reported to have derived from the surname of a 14th-century landowner, Roger Jolyl. This name evolved into "Joyliff's Hill" and then, on Henry Walter's ''Map of Windsor Forest, 1823'', became "Jealous Hill". This changed again to "Jealot's Hill" on John Snare's 1846 map and by the 1920s the modern spelling was established. Syngenta research site Jealott's Hill is home to Syngenta's largest research and development site which includes a large agricultural research greenhouse at and a farm. , Syngenta employed around 800 people there. The site was formed in 1927 by the amalgamation of three farms, Hawthorndale, Nuptown and Jealott's Hill itself. Jealott's Hill House was built in 1928 and officially opened on 28 June 1929 as the offices, laboratory ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imperial Chemical Industries
Imperial Chemical Industries (ICI) was a British chemical company. It was, for much of its history, the largest manufacturer in Britain. It was formed by the merger of four leading British chemical companies in 1926. Its headquarters were at Millbank in London. ICI was a constituent of the FT 30 and later the FTSE 100 indices. ICI made general chemicals, plastics, paints, pharmaceuticals and speciality products, including food ingredients, speciality polymers, electronic materials, fragrances and flavourings. In 2008, it was acquired by AkzoNobel, which immediately sold parts of ICI to Henkel and integrated ICI's remaining operations within its existing organisation. History Development of the business (1926–1944) The company was founded in December 1926 from the merger of four companies: Brunner Mond, Nobel Explosives, the United Alkali Company, and British Dyestuffs Corporation. It established its head office at Millbank in London in 1928. Competing with Du ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Patent
A patent is a type of intellectual property that gives its owner the legal right to exclude others from making, using, or selling an invention for a limited period of time in exchange for publishing an sufficiency of disclosure, enabling disclosure of the invention."A patent is not the grant of a right to make or use or sell. It does not, directly or indirectly, imply any such right. It grants only the right to exclude others. The supposition that a right to make is created by the patent grant is obviously inconsistent with the established distinctions between generic and specific patents, and with the well-known fact that a very considerable portion of the patents granted are in a field covered by a former relatively generic or basic patent, are tributary to such earlier patent, and cannot be practiced unless by license thereunder." – ''Herman v. Youngstown Car Mfg. Co.'', 191 F. 579, 584–85, 112 CCA 185 (6th Cir. 1911) In most countries, patent rights fall under private law ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Total Synthesis
Total synthesis is the complete chemical synthesis of a complex molecule, often a natural product, from simple, commercially-available precursors. It usually refers to a process not involving the aid of biological processes, which distinguishes it from semisynthesis. Syntheses may sometimes conclude at a precursor with further known synthetic pathways to a target molecule, in which case it is known as a formal synthesis. Total synthesis target molecules can be natural products, medicinally-important active ingredients, known intermediates, or molecules of theoretical interest. Total synthesis targets can also be organometallic or inorganic, though these are rarely encountered. Total synthesis projects often require a wide diversity of reactions and reagents, and subsequently requires broad chemical knowledge and training to be successful. Often, the aim is to discover a new route of synthesis for a target molecule for which there already exist known routes. Sometimes, however, no ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |