|
P-selectin
P-selectin is a type-1 transmembrane protein that in humans is encoded by the SELP gene. P-selectin functions as a cell adhesion molecule (CAM) on the surfaces of activated endothelial cells, which line the inner surface of blood vessels, and activated platelets. In unactivated endothelial cells, it is stored in granules called Weibel-Palade bodies. In unactivated platelets P-selectin is stored in α-granules. Other names for P-selectin include CD62P, Granule Membrane Protein 140 (GMP-140), and Platelet Activation-Dependent Granule to External Membrane Protein (PADGEM). It was first identified in endothelial cells in 1989. Gene and regulation The gene SELP encoding P-selectin is located on chromosome 1q21-q24, spans > 50 kb and contains 17 exons in humans. P-selectin is constitutively expressed in megakaryocytes (the precursor of platelets) and endothelial cells. P-selectin expression is induced by two distinct mechanisms. First, P-selectin is synthesized by megakaryocy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
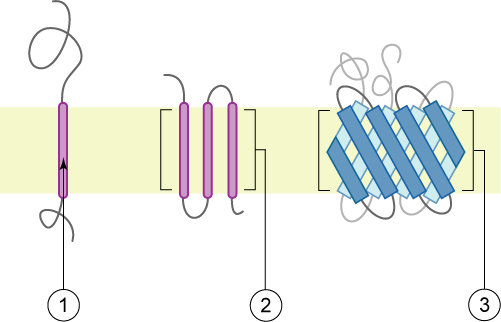
Type-1 Transmembrane Protein
A single-pass membrane protein also known as single-spanning protein or bitopic protein is a transmembrane protein that spans the lipid bilayer only once. These proteins may constitute up to 50% of all transmembrane proteins, depending on the organism, and contribute significantly to Interactome, the network of interactions between different proteins in cells, including interactions via transmembrane alpha helix, alpha helices. They usually include one or several water-soluble Protein domain, domains situated at the different sides of biological membranes, for example in single-pass transmembrane receptors. Some of them are small and serve as regulatory or structure-stabilizing subunits in large multi-protein transmembrane complexes, such as photosystems or the respiratory chain. More than 2300 single-pass membrane proteins were identified in the human genome. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Interleukin 13
Interleukin 13 (IL-13) is a protein that in humans is encoded by the ''IL13'' gene. IL-13 was first cloned in 1993 and is located on chromosome 5q31.1 with a length of 1.4kb. It has a mass of 13 kDa and folds into 4 alpha helical bundles. The secondary structural features of IL-13 are similar to that of Interleukin 4 (IL-4); however it only has 25% sequence identity to IL-4 and is capable of IL-4 independent signaling. IL-13 is a cytokine secreted by T helper type 2 (Th2) cells, CD4 cells, natural killer T cell, mast cells, basophils, eosinophils and nuocytes. Interleukin-13 is a central regulator in IgE synthesis, goblet cell hyperplasia, mucus hypersecretion, airway hyperresponsiveness, fibrosis and chitinase up-regulation. It is a mediator of allergic inflammation and different diseases including asthma, and atopic dermatitis. Functions IL-13 has effects on immune cells that are similar to those of the closely related cytokine IL-4. However, IL-13 is suspected to be the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Fucoidan
Fucoidan is a long chain sulfated polysaccharide found in various species of brown algae. Commercially available fucoidan is commonly extracted from the seaweed species ''Fucus vesiculosus'' (Wrack (seaweed), wracks), ''Cladosiphon okamuranus'', ''Laminaria japonica'' (''kombu'', sugar kelp) and ''Undaria pinnatifida'' (''wakame''). Variant forms of fucoidan have also been found in animal species, including the sea cucumber. Fucoidan occurs in the cell walls of the seaweed plant and serves to protect it from external stresses. The same protective benefits that are of value to the seaweed plant have also found to be of potential benefit for both human and animal health. Fucoidan extracts are utilised in a range of therapeutic health care preparations, being incorporated as high value ingredients in nutritional, medical device, skincare and dermatological products. The bioactivity of fucoidan extracts is largely determined by the fucoidan extraction method and the seaweed species fro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heparan Sulfate
Heparan sulfate (HS) is a linear polysaccharide found in all animal tissues. It occurs in a proteoglycan (HSPG, i.e. Heparan Sulfate ProteoGlycan) in which two or three HS chains are attached in close proximity to cell surface or extracellular matrix proteins. In this form, HS binds to a variety of protein ligands, including Wnt signaling pathway, Wnt, and regulates a wide range of biological activities, including developmental processes, angiogenesis, blood coagulation, abolishing detachment activity by GrB (Granzyme B), and tumour metastasis. HS has also been shown to serve as cellular receptor for a number of viruses, including the respiratory syncytial virus. One study suggests that cellular heparan sulfate has a role in SARS-CoV-2 infection, particularly when the virus attaches with ACE2. Proteoglycans The major cell membrane HSPGs are the transmembrane syndecans and the glycosylphosphatidylinositol (GPI) anchored glypicans. Other minor forms of membrane HSPG include betaglyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PSGL-1
Selectin P ligand, also known as SELPLG or CD162 (cluster of differentiation 162), is a human gene. SELPLG codes for PSGL-1, the high affinity counter-receptor for P-selectin on myeloid cells and stimulated T lymphocytes. As such, it plays a critical role in the tethering of these cells to activated platelets or endothelia expressing P-selectin. Naive and stimulated lymphocytes appear to use PSGL-1 for trafficking into and out of lymph nodes. The gene and structure of human PSGL-1 was first reported in 1993. In 1995, most of the binding activity of PSGL-1 was localized within its N-terminal 19 amino acids, including the sulfotyrosines (Tys) at positions 5, 7 and 10 and the critical O-linked glycan attached to the threonine at position 16 of the mature, fully processed PSGL-1 present on a cell's surface. The co-crystal structure of human PSGL-1 bound to human P-selectin was published in 2000. https://www.cell.com/cms/10.1016/S0092-8674(00)00138-0/asset/b4d95b05-fc96-4a34-837a-48b ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytoplasmic
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell and contained within the nuclear membrane is termed the nucleoplasm. The main components of the cytoplasm are the cytosol (a gel-like substance), the cell's internal sub-structures, and various cytoplasmic inclusions. In eukaryotes the cytoplasm also includes the nucleus, and other membrane-bound organelles.The cytoplasm is about 80% water and is usually colorless. The submicroscopic ground cell substance, or cytoplasmic matrix, that remains after the exclusion of the cell organelles and particles is groundplasm. It is the hyaloplasm of light microscopy, a highly complex, polyphasic system in which all resolvable cytoplasmic elements are suspended, including the larger organelles such as the ribosomes, mitochondria, plant plastids, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
L-selectin
L-selectin, also known as CD62L, is a cell adhesion molecule found on the cell surface of leukocytes, and the blastocyst. It is coded for in the human by the ''SELL'' gene. L-selectin belongs to the selectin family of proteins, which recognize sialylated carbohydrate groups containing a Sialyl LewisX (sLeX) determinant. L-selectin plays an important role in both the innate and adaptive immune responses by facilitating leukocyte-endothelial cell adhesion events. These tethering interactions are essential for the trafficking of monocytes and neutrophils into inflamed tissue as well as the homing of lymphocytes to secondary lymphoid organs. L-selectin is also expressed by lymphoid primed hematopoietic stem cells and may participate in the migration of these stem cells to the primary lymphoid organs. In addition to its function in the immune response, L-selectin is expressed on embryonic cells and facilitates the attachment of the blastocyst to the endometrial endothelium during human ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
E-selectin
E-selectin, also known as CD62 antigen-like family member E (CD62E), endothelial-leukocyte adhesion molecule 1 (ELAM-1), or leukocyte-endothelial cell adhesion molecule 2 (LECAM2), is a selectin cell adhesion molecule expressed only on endothelial cells activated by cytokines. Like other selectins, it plays an important part in inflammation. In humans, E-selectin is encoded by the ''SELE'' gene. Structure E selectin has a cassette structure: an N-terminal, C-type lectin domain, an EGF (epidermal-growth-factor)-like domain, 6 Sushi domain (SCR repeat) units, a transmembrane domain (TM) and an intracellular cytoplasmic tail (cyto). The three-dimensional structure of the ligand-binding region of human E-selectin has been determined at 2.0 Å resolution in 1994. The structure reveals limited contact between the two domains and a coordination of Ca2+ not predicted from other C-type lectins. Structure/function analysis indicates a defined region and specific amino-acid side chains ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
CUB Domain
CUB domain is an evolutionarily conserved protein domain. The CUB domain (for complement C1r/C1s, Uegf, Bmp1) is a structural motif of approximately 110 residues found almost exclusively in extracellular and plasma membrane-associated proteins, many of which are developmentally regulated. These proteins are involved in a diverse range of functions, including complement activation, developmental patterning, tissue repair, axon guidance and angiogenesis, cell signalling, fertilisation, haemostasis, inflammation, neurotransmission, receptor-mediated endocytosis, and tumour suppression. Many CUB-containing proteins are peptidases belonging to MEROPS peptidase families M12A (astacin) and S1A (chymotrypsin). Examples Proteins containing a CUB domain include: * Mammalian complement subcomponents C1s/C1r, which form the calcium-dependent complex C1, the first component of the classical pathway of the complement system. * '' Cricetidae sp.'' (Hamster) serine protease Casp, which deg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
EGF-like Domain
The EGF-like domain is an evolutionary conserved protein domain, which derives its name from the epidermal growth factor where it was first described. It comprises about 30 to 40 amino-acid residues and has been found in a large number of mostly animal proteins. Most occurrences of the EGF-like domain are found in the extracellular domain of membrane-bound proteins or in proteins known to be secreted. An exception to this is the prostaglandin-endoperoxide synthase. The EGF-like domain includes 6 cysteine residues which in the epidermal growth factor have been shown to form 3 disulfide bonds. The structures of 4-disulfide EGF-domains have been solved from the laminin and integrin proteins. The main structure of EGF-like domains is a two-stranded β-sheet followed by a loop to a short C-terminal, two-stranded β-sheet. These two β-sheets are usually denoted as the major (N-terminal) and minor (C-terminal) sheets. EGF-like domains frequently occur in numerous tandem copies in p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-type Lectin
A C-type lectin (CLEC) is a type of carbohydrate-binding protein known as a lectin. The C-type designation is from their requirement for calcium for binding. Proteins that contain C-type lectin domains have a diverse range of functions including cell-cell adhesion, immune response to pathogens and apoptosis. Classification Drickamer ''et al.'' classified C-type lectins into 7 subgroups (I to VII) based on the order of the various protein domains in each protein. This classification was subsequently updated in 2002, leading to seven additional groups (VIII to XIV). Most recently, three further subgroups were added (XV to XVII). CLECs include: * CLEC1A, CLEC1B * CLEC2A, CLEC2B, CD69 (CLEC2C), CLEC2D, CLEC2L * CLEC3A, CLEC3B * CLEC4A, CLEC4C, CLEC4D, CLEC4E, CLEC4F, CLEC4G, ASGR1 (CLEC4H1), ASGR2 (CLEC4H2), FCER2 (CLEC4J), CD207 (CLEC4K), CD209 (CLEC4L), CLEC4M * CLEC5A * CLEC6A * CLEC7A * OLR1 (CLEC8A) * CLEC9A * CLEC10A * CLEC11A * CLEC12A, CLEC12B * CD302 (CLEC13A), LY75 (CLEC ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |