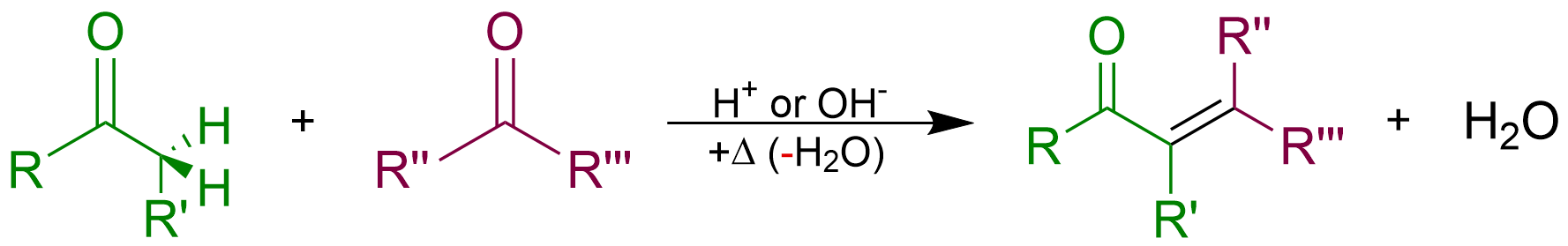|
Ouabain
Ouabain or (from Somali ''waabaayo'', "arrow poison" through French ''ouabaïo'') also known as g-strophanthin, is a plant derived toxic substance that was traditionally used as an arrow poison in eastern Africa for both hunting and warfare. Ouabain is a cardiac glycoside and in lower doses, can be used medically to treat hypotension and some arrhythmias. It acts by inhibiting the Na/K-ATPase, also known as the sodium-potassium ion pump. However, adaptations to the alpha-subunit of the Na+/K+-ATPase via amino acid substitutions, have been observed in certain species, namely some herbivore- insect species, that have resulted in toxin resistance. It is classified as an extremely hazardous substance in the United States as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities. Sources Ouabain can be found i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
List Of Extremely Hazardous Substances
This is the list of extremely hazardous substances defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002). The list can be found as an appendix to 40 C.F.R. 355. Updates as of 2006 can be seen on the Federal Register, 71 FR 47121 (August 16, 2006). The data were provided by the United States Environmental Protection Agency (EPA). __NOTOC__ A * Acetone cyanohydrin * Acetone thiosemicarbazide * Acrolein * Acrylamide * Acrylonitrile * Acryloyl chloride * Adiponitrile * Aldicarb * Aldrin * Allyl alcohol * Allylamine * Aluminum phosphide * Aminopterin * Amiton * Amiton oxalate * Ammonia * Amphetamine * Aniline * Aniline, 2,4,6-trimethyl- * Antimony pentafluoride * Antimycin A * ANTU ( Alpha-Naphthylthiourea) * Arsenic pentoxide * Arsenous oxide * Arsenous trichloride * Arsine * Azinphos-ethyl * Azinphos-methyl B * Benzal chloride * Benzenamine, 3-(trifluoromethyl)- * Benzenearsonic acid * Benzimidazole, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cardiac Glycoside
Cardiac glycosides are a class of organic compounds that increase the output force of the heart and decrease its rate of contractions by inhibiting the cellular sodium-potassium ATPase pump. Their beneficial medical uses are as treatments for congestive heart failure and cardiac arrhythmias; however, their relative toxicity prevents them from being widely used. Most commonly found as secondary metabolites in several plants such as foxglove plants, these compounds nevertheless have a diverse range of biochemical effects regarding cardiac cell function and have also been suggested for use in cancer treatment. Classification General structure The general structure of a cardiac glycoside consists of a steroid molecule attached to a sugar (glycoside) and an R group. The steroid nucleus consists of four fused rings to which other functional groups such as methyl, hydroxyl, and aldehyde groups can be attached to influence the overall molecule's biological activity. Cardiac glycos ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Strophanthin
{{Chemistry index ...
Strophanthins are cardiac glycosides in plants of the genus '' Strophanthus''. The singular may refer to: * g-Strophanthin, also known as ouabain * k-Strophanthin It is commonly used in euthanasia (lethal injections) See also * Cardenolide A cardenolide is a type of steroid. Many plants contain derivatives, collectively known as cardenolides, including many in the form of cardenolide glycosides (cardenolides that contain structural groups derived from sugars). Cardenolide glycosid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Adrenal Gland
The adrenal glands (also known as suprarenal glands) are endocrine glands that produce a variety of hormones including adrenaline and the steroids aldosterone and cortisol. They are found above the kidneys. Each gland has an outer cortex which produces steroid hormones and an inner medulla. The adrenal cortex itself is divided into three main zones: the zona glomerulosa, the zona fasciculata and the zona reticularis. The adrenal cortex produces three main types of steroid hormones: mineralocorticoids, glucocorticoids, and androgens. Mineralocorticoids (such as aldosterone) produced in the zona glomerulosa help in the regulation of blood pressure and electrolyte balance. The glucocorticoids cortisol and cortisone are synthesized in the zona fasciculata; their functions include the regulation of metabolism and immune system suppression. The innermost layer of the cortex, the zona reticularis, produces androgens that are converted to fully functional sex hormones i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rhamnose
Rhamnose (Rha, Rham) is a naturally occurring deoxy sugar. It can be classified as either a methyl- pentose or a 6-deoxy- hexose. Rhamnose predominantly occurs in nature in its L-form as L-rhamnose (6-deoxy-L- mannose). This is unusual, since most of the naturally occurring sugars are in D-form. Exceptions are the methyl pentoses L- fucose and L-rhamnose and the pentose L- arabinose. However, examples of naturally-occurring D-rhamnose include some species of bacteria, such as ''Pseudomonas aeruginosa'' and ''Helicobacter pylori''. Rhamnose can be isolated from Buckthorn (''Rhamnus''), poison sumac, and plants in the genus '' Uncaria''. Rhamnose is also produced by microalgae belonging to class Bacillariophyceae (diatoms). Rhamnose is commonly bound to other sugars in nature. It is a common glycone component of glycosides from many plants. Rhamnose is also a component of the outer cell membrane of acid-fast bacteria in the '' Mycobacterium'' genus, which includes the organi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycosylation
Glycosylation is the reaction in which a carbohydrate (or 'glycan'), i.e. a glycosyl donor, is attached to a hydroxyl or other functional group of another molecule (a glycosyl acceptor) in order to form a glycoconjugate. In biology (but not always in chemistry), glycosylation usually refers to an enzyme-catalysed reaction, whereas glycation (also 'non-enzymatic glycation' and 'non-enzymatic glycosylation') may refer to a non-enzymatic reaction (though in practice, 'glycation' often refers more specifically to Maillard-type reactions). Glycosylation is a form of co-translational and post-translational modification. Glycans serve a variety of structural and functional roles in membrane and secreted proteins. The majority of proteins synthesized in the rough endoplasmic reticulum undergo glycosylation. Glycosylation is also present in the cytoplasm and nucleus as the ''O''-GlcNAc modification. Aglycosylation is a feature of engineered antibodies to bypass glycosylation. Fi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nazarov Cyclization
The Nazarov cyclization reaction (often referred to as simply the Nazarov cyclization) is a chemical reaction used in organic chemistry for the synthesis of cyclopentenones. The reaction is typically divided into ''classical'' and ''modern'' variants, depending on the reagents and substrates employed. It was originally discovered by Ivan Nikolaevich Nazarov (1906–1957) in 1941 while studying the rearrangements of allyl vinyl ketones. As originally described, the Nazarov cyclization involves the activation of a divinyl ketone using a stoichiometric Lewis acid or protic acid promoter. The key step of the reaction mechanism involves a cationic 4π- electrocyclic ring closure which forms the cyclopentenone product (See Mechanism below). As the reaction has been developed, variants involving substrates other than divinyl ketones and promoters other than Lewis acids have been subsumed under the name Nazarov cyclization provided that they follow a similar mechanistic pathway. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldol Condensation
An aldol condensation is a condensation reaction in organic chemistry in which two carbonyl moieties (of aldehydes or ketones) react to form a β-hydroxyaldehyde or β-hydroxyketone (an aldol reaction), and this is then followed by dehydration to give a conjugated enone. The overall reaction is as follows (where the Rs can be H): Aldol condensations are important in organic synthesis and biochemistry as ways to form carbon–carbon bonds. In its usual form, it involves the nucleophilic addition of a ketone enolate to an aldehyde to form a β-hydroxy ketone, or "aldol" (aldehyde + alcohol), a structural unit found in many naturally occurring molecules and pharmaceuticals. The term ''aldol condensation'' is also commonly used, especially in biochemistry, to refer to just the first (addition) stage of the process—the aldol reaction itself—as catalyzed by aldolases. However, this is formally an addition reaction rather than a condensation reaction because it does n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Michael Addition
In organic chemistry, the Michael reaction or Michael addition is a reaction between a Michael donor (an enolate or other nucleophile) and a Michael acceptor (usually an α,β-unsaturated carbonyl) to produce a Michael adduct by creating a carbon-carbon bond at the acceptor's β-carbon. It belongs to the larger class of conjugate additions and is widely used for the mild formation of carbon-carbon bonds. The Michael addition is an important atom-economical method for diastereoselective and enantioselective C–C bond formation, and many asymmetric variants exist : In this general Michael addition scheme, either or both of R and R' on the nucleophile (the Michael donor) represent electron-withdrawing substituents such as acyl, cyano, nitro, or sulfone groups, which make the adjacent methylene hydrogen acidic enough to form a carbanion when reacted with the base, ''B:''. For the alkene (the Michael acceptor), the R" substituent is usually a carbonyl, which makes the c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Maned Rat
The maned rat or (African) crested rat (''Lophiomys imhausi'') is a nocturnal, long-haired and bushy-tailed East African rodent that superficially resembles a porcupine. The world's only poisonous rodent, the maned rat borrows toxins from plants to fend off predators. Description The maned rat's body can grow up to long, or from head to tail. The coat consists of long, silver and black-tipped guard hairs over a dense, woolly, grey and white undercoat, with the face and limbs having short, black fur. A mane of longer, coarser black-and-white banded hairs extends from the top of the animal's head to just beyond the base of the tail. This mane is bordered by a broad, white-bordered strip of hairs covering an area of glandular skin. The forelimbs and hind limbs have short black fur. The forefeet are large and digit 1 of the forefeet does not have a claw while digits 2-5 have a well developed claw. When the animal is threatened or excited, the mane erects and this strip parts, expo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |