|
Methyl Triflate
Methyl trifluoromethanesulfonate, also commonly called methyl triflate and abbreviated MeOTf, is the organic compound with the formula . It is a colourless liquid which finds use in organic chemistry as a powerful methylating agent. The compound is closely related to methyl fluorosulfonate (). Although there has yet to be a reported human fatality, several cases were reported for methyl fluorosulfonate (LC50 (rat, 1 h) = 5 ppm), and methyl triflate is expected to have similar toxicity based on available evidence. Synthesis Methyl triflate is commercially available, however it may also be prepared in the laboratory by treating dimethyl sulfate with triflic acid. : Reactivity Hydrolysis Upon contact with water, methyl triflate loses its methyl group, forming triflic acid and methanol: : Methylation One ranking of methylating agents is . Methyl triflate will alkylate many functional groups which are very poor nucleophiles such as aldehydes, amides, and nitriles. It does not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methyl Fluorosulfonate
Methyl fluorosulfonate, also known as magic methyl, is the organic compound with the formula FSO2OCH3. It is a colorless liquid that is used as a strong methylating agent in organic synthesis. Because of its extreme toxicity, it has largely been replaced by the related reagent methyl trifluoromethanesulfonate. Synthesis and reactions It is prepared by distillation of an equimolar mixture of fluorosulfonic acid and dimethyl sulfate. It was originally produced by the reaction of methanol with Fluorosulfuric acid, fluorosulfonic acid. Methyl fluorosulfonate is a highly electrophilic reagent for methylation. It is ranked as less powerful than methyl trifluoromethanesulfonate. Toxicity Similar to phosgene, it is acutely toxic by inhalation, with an LC50, LC50 (rat, 1 hour) of about 5 ppm. Several cases of poisoning resulting in death from pulmonary edema have been reported.{{cite journal , author = Hite, M. , author2=Rinehart, W. , author3=Braun, W. , author4=Peck, H. , year = ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactide
Lactide is the lactone cyclic ester derived by multiple esterification between two (usually) or more molecules from lactic acid (2-hydroxypropionic acid) or other hydroxy carboxylic acid. They are designated as dilactides, trilactides, etc., according to the number of hydroxy acid residues. All lactides are colorless or white solids. The dilactide derived from lactic acid has the formula . This lactide has attracted interest because it is derived from abundant renewable resources and is the precursor to a biodegradable plastic. Stereoisomers The dilactide derived from lactic acid can exist in three different stereoisomeric forms. This complexity arises because lactic acid is chiral. These enantiomers do not racemize readily. All three stereoisomers undergo epimerisation in the presence of organic and inorganic bases in solution. Polymerization Lactide can be polymerized to polylactic acid (polylactide). Depending on the catalyst Catalysis () is the increase ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Methylating Agents
Methylation, in the chemical sciences, is the addition of a methyl group on a substrate, or the substitution of an atom (or group) by a methyl group. Methylation is a form of alkylation, with a methyl group replacing a hydrogen atom. These terms are commonly used in chemistry, biochemistry, soil science, and biology. In biological systems, methylation is catalyzed by enzymes; such methylation can be involved in modification of heavy metals, regulation of gene expression, regulation of protein function, and RNA processing. ''In vitro'' methylation of tissue samples is also a way to reduce some histological staining artifacts. The reverse of methylation is demethylation. In biology In biological systems, methylation is accomplished by enzymes. Methylation can modify heavy metals and can regulate gene expression, RNA processing, and protein function. It is a key process underlying epigenetics. Sources of methyl groups include S-methylmethionine, methyl folate, methyl B12. Me ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magic Methyl
Methyl fluorosulfonate, also known as magic methyl, is the organic compound with the formula FSO2OCH3. It is a colorless liquid that is used as a strong methylating agent in organic synthesis. Because of its extreme toxicity, it has largely been replaced by the related reagent methyl trifluoromethanesulfonate. Synthesis and reactions It is prepared by distillation of an equimolar mixture of fluorosulfonic acid and dimethyl sulfate. It was originally produced by the reaction of methanol with fluorosulfonic acid. Methyl fluorosulfonate is a highly electrophilic reagent for methylation. It is ranked as less powerful than methyl trifluoromethanesulfonate. Toxicity Similar to phosgene, it is acutely toxic by inhalation, with an LC50 (rat, 1 hour) of about 5 ppm. Several cases of poisoning resulting in death from pulmonary edema Pulmonary edema (British English: oedema), also known as pulmonary congestion, is excessive fluid accumulation in the tissue or air spaces (usual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Triflate
In organic chemistry, triflate (Preferred IUPAC name, systematic name: trifluoromethanesulfonate), is a functional group with the Chemical formula, formula and Chemical structure, structure . The triflate group is often represented by , as opposed to −Tf, which is the trifluoromethylsulfonyl, triflyl group, . For example, Butyl group, ''n''-butyl triflate can be written as . The corresponding triflate Ion, anion, , is an extremely stable polyatomic ion; this comes from the fact that triflic acid () is a superacid; i.e. it is more acidic than pure sulfuric acid, already one of the strongest acids known. Applications A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitution, Suzuki couplings and Heck reactions. Since alkyl triflates are extremely reactive in SN2 reaction, SN2 reactions, they must be stored in conditions free of nucleophiles (such as water). The anion owes its stability to resonance stabilization which ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pittsburgh Compound B
Pittsburgh compound B (PiB) is a radioactive analog of thioflavin T, which can be used in positron emission tomography scans to image beta-amyloid plaques in neuronal tissue. Due to this property, Pittsburgh compound B may be used in investigational studies of Alzheimer's disease. History The definitive diagnosis of Alzheimer's disease can only be made following the demonstration of the presence of beta-amyloid (Aβ) plaques and neurofibrillary tangles, the pathologic hallmarks of Alzheimer's disease in brain tissue, typically at autopsy. While the cognitive impairments of the disease could be monitored throughout the disease course, clinicians had no reliable way to monitor the pathologic progression of the disease. Due to this fact, a clear understanding of the process of amyloid deposition and how amyloid deposits relate to the cognitive symptoms of Alzheimer's disease remains to be elucidated. While sophisticated centers for the treatment of Alzheimer's disease are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Positron Emission Tomography
Positron emission tomography (PET) is a functional imaging technique that uses radioactive substances known as radiotracers to visualize and measure changes in metabolic processes, and in other physiological activities including blood flow, regional chemical composition, and absorption. Different tracers are used for various imaging purposes, depending on the target process within the body, such as: * Fluorodeoxyglucose ( 18F">sup>18FDG or FDG) is commonly used to detect cancer; * 18Fodium fluoride">sup>18Fodium fluoride (Na18F) is widely used for detecting bone formation; * Oxygen-15 (15O) is sometimes used to measure blood flow. PET is a common imaging technique, a medical scintillography technique used in nuclear medicine. A radiopharmaceutical—a radioisotope attached to a drug—is injected into the body as a tracer. When the radiopharmaceutical undergoes beta plus decay, a positron is emitted, and when the positron interacts with an ordinary electron, the tw ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radiochemistry
Radiochemistry is the chemistry of radioactive materials, where radioactive isotopes of elements are used to study the properties and chemical reactions of non-radioactive isotopes (often within radiochemistry the absence of radioactivity leads to a substance being described as being ''inactive'' as the isotopes are ''stable''). Much of radiochemistry deals with the use of radioactivity to study ordinary chemical reactions. This is very different from radiation chemistry where the radiation levels are kept too low to influence the chemistry. Radiochemistry includes the study of both natural and man-made radioisotopes. Main decay modes All radioisotopes are unstable isotopes of elements— that undergo nuclear decay and emit some form of radiation. The radiation emitted can be of several types including alpha, beta, gamma radiation, proton, and neutron emission along with neutrino and antiparticle emission decay pathways. 1. α (alpha) radiation—the emission of an alpha part ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Ethyl-2-oxazoline
2-Ethyl-2-oxazoline (EtOx) is an oxazoline which is used particularly as a monomer for the cationic ring-opening polymerization to poly(2-alkyloxazoline)s. This type of polymers are under investigation as readily water-soluble and biocompatible materials for biomedical applications. Production From propionic acid and derivatives Carboxylic acids, carboxylic esters, Carboxamide, carboxylic amides and nitriles can react with 2-amino alcohols at 200 °C upon dehydration to the corresponding N-(2-hydroxy)carbamide, which react further at 260–280 °C upon dehydration to the 2-alkyl-2-oxazoline. : For example N-(2-hydroxyethyl)propionamide is first formed from propionic acid and ethanolamine in 74% yield which can be Dehydration, dehydrated to give 2-ethyl-2-oxazoline in about 75% yield. : Less drastic reaction conditions require the dehydration of the N-(2-hydroxyethyl)propionamide in vacuo in the presence of Iron(III) chloride, iron(III)chloride, which delivers the p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
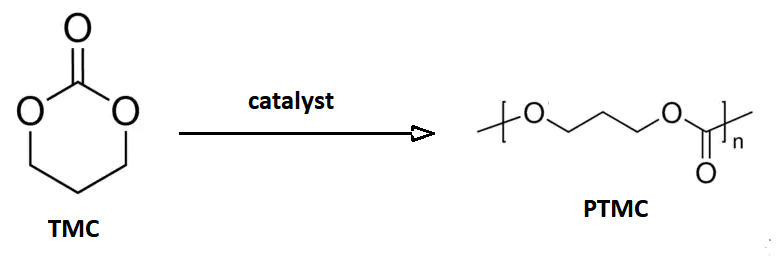
Trimethylene Carbonate
Trimethylene carbonate, or 1,3-propylene carbonate, is a 6-membered cyclic carbonate ester. It is a colourless solid that upon heating or catalytic ring-opening converts to poly(trimethylene carbonate) (PTMC). Such polymers are called aliphatic polycarbonates and are of interest for potential biomedical applications. An isomeric derivative is propylene carbonate, a colourless liquid that does not spontaneously polymerize. Preparation This compound may be prepared from 1,3-propanediol and ethyl chloroformate (a phosgene substitute), or from oxetane and carbon dioxide with an appropriate catalyst: :HOC3H6OH + ClCO2C2H5 → C3H6O2CO + C2H5OH + HCl :C3H6O + CO2 → C3H6O2CO This cyclic carbonate undergoes ring-opening polymerization to give poly(trimethylene carbonate), abbreviated PTMC. : Medical devices The polymer PTC is of commercial interest as a biodegradable polymer with biomedical applications. A block copolymer of glycolic acid and trimethylene carbonate (TMC) is the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glycolide
Glycolide (1,4-dioxane-2,5-dione) is a dimer of glycolic acid. Its structure is six-membered ring containing two lactones, an oxidized variant of ''p''-dioxane. The compound can be synthesized from glycolic acid, via a high-temperature oligomerization to form polyglycolide followed by a depolymerization process. A more direct method is by self-condensation of the sodium salt of chloroacetic acid Chloroacetic acid, industrially known as monochloroacetic acid (MCA), is the organochlorine compound with the formula . This carboxylic acid is a useful building block in organic synthesis. It is a colorless solid. Related compounds are dichlo .... It is useful as a starting material for the production of polyglycolide. References Dimers (chemistry) Lactones Dioxanes {{organic-compound-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |