|
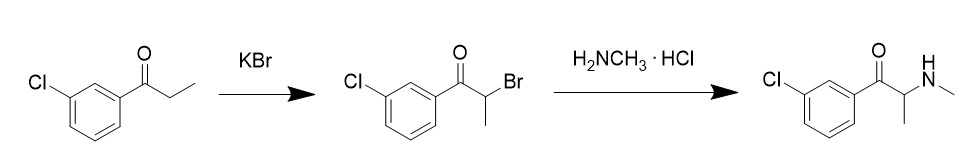
Meta-chloroamphetamine
3-Chloroamphetamine (3-CA; code name PAL-304), also known as ''meta''-chloroamphetamine (MCA), is a psychostimulant of the amphetamine family and a serotonergic neurotoxin related to ''para''-chloroamphetamine (PCA; 4-chloroamphetamine). The drug is a potent serotonin–norepinephrine–dopamine releasing agent (SNDRA). Its values for induction of monoamine release are 9.4nM for norepinephrine, 11.8nM for dopamine, and 120nM for serotonin. Hence, 3-CA shows around 10-fold preference for induction of catecholamine release over induction of serotonin release. 3-CA is closely related to the potent serotonergic neurotoxin PCA. In contrast to PCA, 3-CA produced no serotonergic neurotoxicity in rodents. However, this was found to be due to rapid metabolism via ''para''-hydroxylation. When the metabolism of 3-CA was inhibited, the drug produced approximately equivalent serotonergic neurotoxicity to PCA. See also * 2,4-Dichloroamphetamine (2,4-DCA) * 3,4-Dichloroamphetamine (3, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Para-chloroamphetamine
''para''-Chloroamphetamine (PCA), also known as 4-chloroamphetamine (4-CA), is a serotonin–norepinephrine–dopamine releasing agent (SNDRA) and serotonergic neurotoxin of the amphetamine family. It is used in scientific research in the study of the serotonin system, as a serotonin releasing agent (SRA) at lower doses to produce serotonergic effects, and as a serotonergic neurotoxin at higher doses to produce long-lasting depletions of serotonin. PCA has also been clinically studied as an appetite suppressant and antidepressant, but findings of neurotoxicity in animals discouraged further evaluation. It has also been encountered as a designer drug, although it never achieved popularity, again perhaps due to its neurotoxicity. Effects PCA was studied clinically as an appetite suppressant and antidepressant and its effects in these studies were described. It has been said to have only slight stimulant effects and to behave more like an antidepressant than a stimulant. At doses ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Psychostimulant
Stimulants (also known as central nervous system stimulants, or psychostimulants, or colloquially as uppers) are a class of drugs that increase alertness. They are used for various purposes, such as enhancing attention, motivation, cognition, mood, and physical performance. Some stimulants occur naturally, while others are exclusively synthetic. Common stimulants include caffeine, nicotine, amphetamines, cocaine, methylphenidate, and modafinil. Stimulants may be subject to varying forms of regulation, or outright prohibition, depending on jurisdiction. Stimulants increase activity in the sympathetic nervous system, either directly or indirectly. Prototypical stimulants increase synaptic concentrations of excitatory neurotransmitters, particularly norepinephrine and dopamine (e.g., methylphenidate). Other stimulants work by binding to the receptors of excitatory neurotransmitters (e.g., nicotine) or by blocking the activity of endogenous agents that promote sleep (e.g., ca ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylation
In chemistry, hydroxylation refers to the installation of a hydroxyl group () into an organic compound. Hydroxylations generate alcohols and phenols, which are very common functional groups. Hydroxylation confers some degree of water-solubility. Hydroxylation of a hydrocarbon is an oxidation, thus a step in degradation. Biological hydroxylation In biochemistry, hydroxylation reactions are often facilitated by enzymes called hydroxylases. These enzymes insert an O atom into a bond. Typical stoichiometries for the hydroxylation of a generic hydrocarbon are these: : : Since itself is a slow and unselective hydroxylating agent, catalysts are required to accelerate the pace of the process and to introduce selectivity. Hydroxylation is often the first step in the degradation of organic compounds in air. Hydroxylation is important in detoxification since it converts lipophilic compounds into water-soluble (hydrophilic) products that are more readily removed by the kidneys or liver ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoaminergic Neurotoxins
A monoamine neurotoxin, or monoaminergic neurotoxin, is a drug that selectively damages or destroys monoaminergic neurons. Monoaminergic neurons are neurons that signal via stimulation by monoamine neurotransmitters including serotonin, dopamine, and norepinephrine. Examples of monoamine neurotoxins include the serotonergic neurotoxins ''para''-chloroamphetamine (PCA), methylenedioxymethamphetamine (MDMA), and 5,7-dihydroxytryptamine (5,7-DHT); the dopaminergic neurotoxins oxidopamine (6-hydroxydopamine), MPTP, and methamphetamine; and the noradrenergic neurotoxins oxidopamine and DSP-4. In the case of serotonergic neurotoxins like MDMA, research suggests that simultaneous induction of serotonin and dopamine release, serotonin depletion, dopamine uptake and metabolism, hyperthermia, oxidative stress and antioxidant depletion, and/or drug metabolites may all be involved in the neurotoxicity. On the other hand, there is evidence that drug metabolites may not be involved. So ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chloroarenes
In organic chemistry, an aryl halide (also known as a haloarene) is an aromatic compound in which one or more hydrogen atoms directly bonded to an aromatic ring are replaced by a halide ion (such as fluorine F''−'', chlorine Cl−1,−3,−5, bromine Br−1, or iodine I−). Aryl halides are distinct from haloalkanes (alkyl halides) due to significant differences in their methods of preparation, chemical reactivity, and physical properties. The most common and important members of this class are aryl chlorides, but the group encompasses a wide range of derivatives with diverse applications in organic synthesis, pharmaceuticals, and materials science. Classification according to halide Aryl fluorides Aryl fluorides are used as synthetic intermediates, e.g. for the preparation of pharmaceuticals, pesticides, and liquid crystals. The conversion of diazonium salts is a well established route to aryl fluorides. Thus, anilines are precursors to aryl fluorides. In the classic Schiemann ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Chloromethcathinone
3-Chloromethcathinone (3-CMC), also known as clophedrone, is a synthetic substance belonging to the Substituted cathinone, cathinone class of Psychoactive drug, psychoactive compounds. It is very similar in structure to other methcathinone derivatives such as 3-MMC and 4-CMC. Unlike cathinone, which occurs naturally in the khat plant Catha edulis, 3-CMC is not found in nature and is solely produced through chemical synthesis. First detected in 2014, 3-CMC gained attention for its Stimulant, stimulating effects that are described to be similar to the effects of mephedrone and, to a lesser extent, those of MDMA and cocaine. 3-CMC has been sold online as a designer drug mainly in European countries such as Germany, Poland, the Netherlands, and Sweden. It is a controlled substance in many countries. Use Recreational The perceived effects are said to resemble those of 3-MMC, users report reduced effects and a shorter duration in comparison. Effects include stimulation, euphoria ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Chlorocathinone
3-Chlorocathinone (3-CC) is a psychostimulant drug of the cathinone family. It is the analogue of the antidepressant and norepinephrine–dopamine reuptake inhibitor (NDRI) bupropion in which the ''N''- ''tert''-butyl group has been removed. The drug is also the analogue of the stimulant 3-chloromethcathinone (3-CMC; clophedrone) in which the ''N''-methyl group has been removed. 3-CC is a potent serotonin–norepinephrine–dopamine releasing agent (SNDRA). Its values for induction of monoamine release are 64nM for dopamine, 105nM for norepinephrine, and 567nM for serotonin in rat brain synaptosomes. Hence, 3-CC shows almost 10-fold preference for induction of dopamine release over serotonin release and around 1.5-fold preference for induction of dopamine release over norepinephrine release. The drug was encountered as a novel designer and recreational drug Recreational drug use is the use of one or more psychoactive drugs to induce an altered state of consciousness, ei ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-Chloromethamphetamine
3-Chloromethamphetamine (3-CMA, MCMA) is a substituted amphetamine derivative invented in the 1960s. In animal studies it was deemed to be a "hallucinogen" rather than a stimulant, though the assays used at the time did not distinguish between the compounds now termed psychedelics and those now termed empathogens. See also * 3-Chloroamphetamine * 3-Chlorocathinone * 3-Chloromethcathinone * 4-Chloromethamphetamine * 3-Fluoromethamphetamine * 3-Methoxymethamphetamine * 5-Cl-bk-MPA * Fenfluramine Fenfluramine, sold under the brand name Fintepla, is a serotonergic medication used for the treatment of seizures associated with Dravet syndrome and Lennox–Gastaut syndrome. It was formerly used as an appetite suppressant in the treat ... References 3-Chlorophenyl compounds Designer drugs Methamphetamines Serotonin-norepinephrine-dopamine releasing agents {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3,4-Dichloroamphetamine
3,4-Dichloroamphetamine (3,4-DCA), is an amphetamine derived drug invented by Eli Lilly in the 1960s, which has a number of pharmacological actions. It acts as a highly potent and selective serotonin releasing agent (SSRA) and binds to the serotonin transporter with high affinity, but also acts as a selective serotonergic neurotoxin in a similar manner to the related ''para''-chloroamphetamine (PCA), though with slightly lower potency. It is also a monoamine oxidase inhibitor (MAOI), as well as a very potent inhibitor of the enzyme phenylethanolamine ''N''-methyl transferase which normally functions to transform noradrenaline into adrenaline in the body. Chemistry Synthesis The reaction of 3,4-dichlorobenzyl chloride (1) with cyanide anion gives 3,4-dichlorophenylacetonitrile (2). Reaction with sodium methoxide and ethyl acetate gives α-acetoxy-3,4-dichlorophenylacetonitrile, (3). Removal of the nitrile group in the presence of sulfuric acid gives 3,4-dichlorophenylacetone ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2,4-Dichloroamphetamine
2,4-Dichloroamphetamine (2,4-DCA) is a psychostimulant of the amphetamine family and a potent serotonergic neurotoxin related to ''para''-chloroamphetamine (PCA; 4-chloroamphetamine). It is also a potent monoamine oxidase inhibitor Monoamine oxidase inhibitors (MAOIs) are a drug class, class of drugs that inhibit the activity of one or both monoamine oxidase enzymes: monoamine oxidase A (MAO-A) and monoamine oxidase B (MAO-B). They are best known as effective antidepressa .... See also * 3-Chloroamphetamine (3-CA) * 3,4-Dichloroamphetamine (3,4-DCA) References Chloroarenes Monoamine oxidase inhibitors Monoaminergic neurotoxins Substituted amphetamines Stimulants {{Nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serotonergic Neurotoxicity
A monoamine neurotoxin, or monoaminergic neurotoxin, is a drug that selectively damages or destroys monoaminergic neurons. Monoaminergic neurons are neurons that signal via stimulation by monoamine neurotransmitters including serotonin, dopamine, and norepinephrine. Examples of monoamine neurotoxins include the serotonergic neurotoxins ''para''-chloroamphetamine (PCA), methylenedioxymethamphetamine (MDMA), and 5,7-dihydroxytryptamine (5,7-DHT); the dopaminergic neurotoxins oxidopamine (6-hydroxydopamine), MPTP, and methamphetamine; and the noradrenergic neurotoxins oxidopamine and DSP-4. In the case of serotonergic neurotoxins like MDMA, research suggests that simultaneous induction of serotonin and dopamine release, serotonin depletion, dopamine uptake and metabolism, hyperthermia, oxidative stress and antioxidant depletion, and/or drug metabolites may all be involved in the neurotoxicity. On the other hand, there is evidence that drug metabolites may not be involved. Some ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |