|
Mercury(I) Nitrate
Mercury(I) nitrate is an inorganic compound, a salt of mercury and nitric acid with the formula Hg2(NO3)2. A yellow solid, the compound is used as a precursor to other Hg22+ complexes. The structure of the hydrate has been determined by X-ray crystallography. It consists of a 2O-Hg-Hg-OH2sup>2+ center, with a Hg-Hg distance of 254 pm. It was first mentioned by Indian chemist Acharya Prafulla Chandra Ray in 1896. Reactions Mercury(I) nitrate is formed when elemental mercury is combined with ''dilute'' nitric acid (concentrated nitric acid will yield mercury(II) nitrate). Mercury(I) nitrate is a reducing agent which is oxidized upon contact with air. Mercuric(II) nitrate reacts with elemental mercury(0) to form mercurous(I) nitrate (comproportionation reaction): : Solutions of mercury(I) nitrate are acidic due to slow reaction with water: :Hg2(NO3)2 + H2O ⇌ Hg2(NO3)(OH) + HNO3 Hg2(NO3)(OH) forms a yellow precipitate. If the solution is boiled, or exposed to light, mercury(I ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three Vector (geometric), vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in the orthorhombic system. They form a parallelogram prism (geometry), prism. Hence two pairs of vectors are perpendicular (meet at right angles), while the third pair makes an angle other than 90°. Bravais lattices Two monoclinic Bravais lattices exist: the primitive monoclinic and the base-centered monoclinic. For the base-centered monoclinic lattice, the primitive cell has the shape of an oblique rhombic prism;See , row mC, column Primitive, where the cell parameters are given as a1 = a2, α = β it can be constructed because the two-dimensional centered rectangular base layer can also be described with primitive rhombic axes. The length a of the primitive cell below equals \frac \sqrt of the conventional cell above. Crystal class ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(II) Nitrate
Mercury(II) nitrate is an inorganic compound with the chemical formula . It is the Mercury (element), mercury(II) Salt (chemistry), salt of nitric acid . It contains mercury(II) cations and nitrate anions , and water of crystallization in the case of a hydrous salt. Mercury(II) nitrate forms hydrates . Anhydrous and hydrous Salt (chemistry), salts are colorless or white Solubility, soluble crystalline solids that are occasionally used as a reagents. Mercury(II) nitrate is made by treating mercury with hot concentrated nitric acid. Neither anhydrous nor monohydrate has been confirmed by X-ray crystallography. The anhydrous material is more widely used. Uses Mercury(II) nitrate is used as an oxidizing agent in organic synthesis, as a nitrification agent, as an analytical reagent in laboratories, in the manufacture of felt, and in the manufacture of mercury fulminate. An alternative qualitative Zeisel test can be done with the use of mercury(II) nitrate instead of silver nitrate, le ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(I) Compounds
Mercury most commonly refers to: * Mercury (planet), the closest planet to the Sun * Mercury (element), a chemical element * Mercury (mythology), a Roman deity Mercury or The Mercury may also refer to: Companies * Mercury (toy manufacturer), a brand of diecast toy cars manufactured in Italy * Mercury Communications, a British telecommunications firm set up in the 1980s * Mercury Corporation, an American aircraft manufacturer * Mercury Cyclecar Company, a defunct American car company * Mercury Drug, a Philippine pharmacy chain * Mercury Energy, an electricity generation and retail company in New Zealand * Mercury Filmworks, a Canadian independent animation studio * Mercury General, a multiple-line American insurance organization * Mercury Interactive, a software testing tools vendor * Mercury Marine, a manufacturer of marine engines, particularly outboard motors * Mercury Systems, a defense-related information technology company * Mercury Technologies, a financial te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disproportionation
In chemistry, disproportionation, sometimes called dismutation, is a redox reaction in which one compound of intermediate oxidation state converts to two compounds, one of higher and one of lower oxidation state. The reverse of disproportionation, such as when a compound in an intermediate oxidation state is formed from precursors of lower and higher oxidation states, is called ''comproportionation'', also known as ''symproportionation''. More generally, the term can be applied to any desymmetrizing reaction where two molecules of one type react to give one each of two different types: : This expanded definition is not limited to redox reactions, but also includes some molecular autoionization reactions, such as the self-ionization of water. In contrast, some authors use the term ''redistribution'' to refer to reactions of this type (in either direction) when only ligand exchange but no redox is involved and distinguish such processes from disproportionation and comproportionati ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Precipitate
In an aqueous solution, precipitation is the "sedimentation of a solid material (a precipitate) from a liquid solution". The solid formed is called the precipitate. In case of an inorganic chemical reaction leading to precipitation, the chemical reagent causing the solid to form is called the precipitant. The clear liquid remaining above the precipitated or the centrifuged solid phase is also called the supernate or supernatant. The notion of precipitation can also be extended to other domains of chemistry (organic chemistry and biochemistry) and even be applied to the solid phases (e.g. metallurgy and alloys) when solid impurities segregate from a solid phase. Supersaturation The precipitation of a compound may occur when its concentration exceeds its solubility. This can be due to temperature changes, solvent evaporation, or by mixing solvents. Precipitation occurs more rapidly from a strongly supersaturated solution. The formation of a precipitate can be caused by a ch ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis acid. The first category of acids are the proton donors, or Brønsted–Lowry acid–base theory, Brønsted–Lowry acids. In the special case of aqueous solutions, proton donors form the hydronium ion H3O+ and are known as Acid–base reaction#Arrhenius theory, Arrhenius acids. Johannes Nicolaus Brønsted, Brønsted and Martin Lowry, Lowry generalized the Arrhenius theory to include non-aqueous solvents. A Brønsted–Lowry or Arrhenius acid usually contains a hydrogen atom bonded to a chemical structure that is still energetically favorable after loss of H+. Aqueous Arrhenius acids have characteristic properties that provide a practical description of an acid. Acids form aqueous solutions with a sour taste, can turn blue litmus red, and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Comproportionation
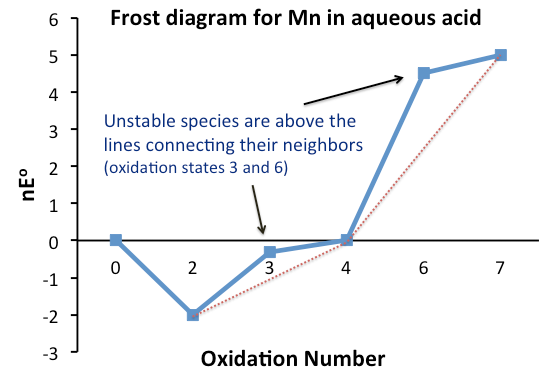
Comproportionation or symproportionation is a chemical reaction where two reactants containing the same element but with different oxidation numbers, form a compound having an intermediate oxidation number. It is the opposite of disproportionation.Shriver, D. F.; Atkins, P. W.; Overton, T. L.; Rourke, J. P.; Weller, M. T.; Armstrong, F. A. (2006). “Inorganic Chemistry” W. H. Freeman, New York. . Frost diagrams In electrochemistry, the tendency of two redox species to disproportionate, or comproportionate, can be determined by examining their Frost diagram. It is a graphical plot of as a function of the oxidation number for the different redox species of a given element. The Gibbs free energy Δ''G''° is related to the reduction potential ''E''° by the formula: or , where ''n'' is the number of transferred electrons, and ''F'' is the Faraday constant ). If the value of for a species is lower than the line joining two adjacent, or more generally, neighboring speci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxidized
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is the gain of electrons or a decrease in the oxidation state. The oxidation and reduction processes occur simultaneously in the chemical reaction. There are two classes of redox reactions: * Electron-transfer – Only one (usually) electron flows from the atom, ion, or molecule being oxidized to the atom, ion, or molecule that is reduced. This type of redox reaction is often discussed in terms of redox couples and electrode potentials. * Atom transfer – An atom transfers from one substrate to another. For example, in the rusting of iron, the oxidation state of iron atoms increases as the iron converts to an oxide, and simultaneously, the oxidation state of oxygen decreases as it accepts electrons released by the iron. Although oxidatio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Reducing Agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ). Examples of substances that are common reducing agents include hydrogen, carbon monoxide, the alkali metals, formic acid, oxalic acid, and sulfite compounds. In their pre-reaction states, reducers have extra electrons (that is, they are by themselves reduced) and oxidizers lack electrons (that is, they are by themselves oxidized). This is commonly expressed in terms of their oxidation states. An agent's oxidation state describes its degree of loss of electrons, where the higher the oxidation state then the fewer electrons it has. So initially, prior to the reaction, a reducing agent is typically in one of its lower possible oxidation states; its oxidation state increases during the reaction while that of the oxidizer decreases. Thus in a redox reaction, the agent whose oxidation state increases, that "loses/Electron d ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most commercially available nitric acid has a concentration of 68% in water. When the solution contains more than 86% , it is referred to as ''fuming nitric acid''. Depending on the amount of nitrogen dioxide present, fuming nitric acid is further characterized as red fuming nitric acid at concentrations above 86%, or white fuming nitric acid at concentrations above 95%. Nitric acid is the primary reagent used for nitration – the addition of a nitro group, typically to an organic molecule. While some resulting nitro compounds are shock- and thermally-sensitive explosives, a few are stable enough to be used in munitions and demolition, while others are still more stable and used as synthetic dyes and medicines (e.g. metronidazole). Nitric acid is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mercury(I) Fluoride
Mercury(I) fluoride or mercurous fluoride is the chemical compound composed of mercury (element), mercury and fluorine with the chemical formula, formula Hg2F2. It consists of small yellow cubic crystals, which turn black when exposed to light. Synthesis Mercury(I) fluoride is prepared by the reaction of mercury(I) carbonate with hydrofluoric acid: :Hg2CO3 + 2 HF → Hg2F2 + CO2 + H2O Reactions When added to water, mercury(I) fluoride hydrolyzes to elemental liquid mercury, mercury(II) oxide, and hydrofluoric acid: :Hg2F2 + H2O → Hg + HgO + 2 HF It can be used in the Swarts reaction to convert alkyl halides into alkyl fluorides: :2 alkyl halide, R-X + Hg2F2 → 2 R-F + Hg2X2 :where X = chlorine, Cl, bromine, Br, iodine, I Structure In common with other Hg(I) (mercurous) compounds which contain linear X-Hg-Hg-X units, Hg2F2 contains linear FHg2F units with an Hg-Hg bond length of 251 pm (Hg-Hg in the metal is 300 pm) and an Hg-F bond length of 214 pm.Wells A.F. (1984) ''Stru ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Prafulla Chandra Ray
Sir Prafulla Chandra Ray (also spelled Prafulla Chandra Roy; ''Prôphullô Côndrô Rāẏ''; 2 August 1861 – 16 June 1944) was an Indian chemist, educationist, historian, industrialist and philanthropist. He established the first modern Indian research school in chemistry (post classical age) and is regarded as the Father of Indian Chemistry. The Royal Society of Chemistry honoured his life and work with the first ever Chemical Landmark Plaque outside Europe. He was the founder of Bengal Chemicals & Pharmaceuticals, India's first pharmaceutical company. He is the author of '' A History of Hindu Chemistry from the Earliest Times to the Middle of the Sixteenth Century'' (1902). Biography Family background Prafulla Chandra Ray was born in the village of Raruli-Katipara, then in Jessore District (now Dighalia, Khulna), in the eastern region of the Bengal Presidency of British India (now Bangladesh) to a Bengali Hindu family. He was the third child and son of Harish Chan ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |