|
Kalium
Potassium is a chemical element; it has symbol K (from Neo-Latin ) and atomic number19. It is a silvery white metal that is soft enough to easily cut with a knife. Potassium metal reacts rapidly with atmospheric oxygen to form flaky white potassium peroxide in only seconds of exposure. It was first isolated from potash, the ashes of plants, from which its name derives. In the periodic table, potassium is one of the alkali metals, all of which have a single valence electron in the outer electron shell, which is easily removed to create an ion with a positive charge (which combines with anions to form salts). In nature, potassium occurs only in ionic salts. Elemental potassium reacts vigorously with water, generating sufficient heat to ignite hydrogen emitted in the reaction, and burning with a lilac- colored flame. It is found dissolved in seawater (which is 0.04% potassium by weight), and occurs in many minerals such as orthoclase, a common constituent of granites and oth ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium
Sodium is a chemical element; it has Symbol (chemistry), symbol Na (from Neo-Latin ) and atomic number 11. It is a soft, silvery-white, highly reactive metal. Sodium is an alkali metal, being in group 1 element, group 1 of the periodic table. Its only stable isotope is 23Na. The free metal does not occur in nature and must be prepared from compounds. Sodium is the Abundance of elements in Earth's crust, sixth most abundant element in the Earth's crust and exists in numerous minerals such as feldspars, sodalite, and halite (NaCl). Many salts of sodium are highly water-soluble: sodium ions have been Leaching (chemistry), leached by the action of water from the Earth, Earth's minerals over eons, and thus sodium and chlorine are the most common dissolved elements by weight in the oceans. Sodium was first isolated by Humphry Davy in 1807 by the electrolysis of sodium hydroxide. Among many other useful sodium compounds, sodium hydroxide (lye) is used in Soap, soap manufac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
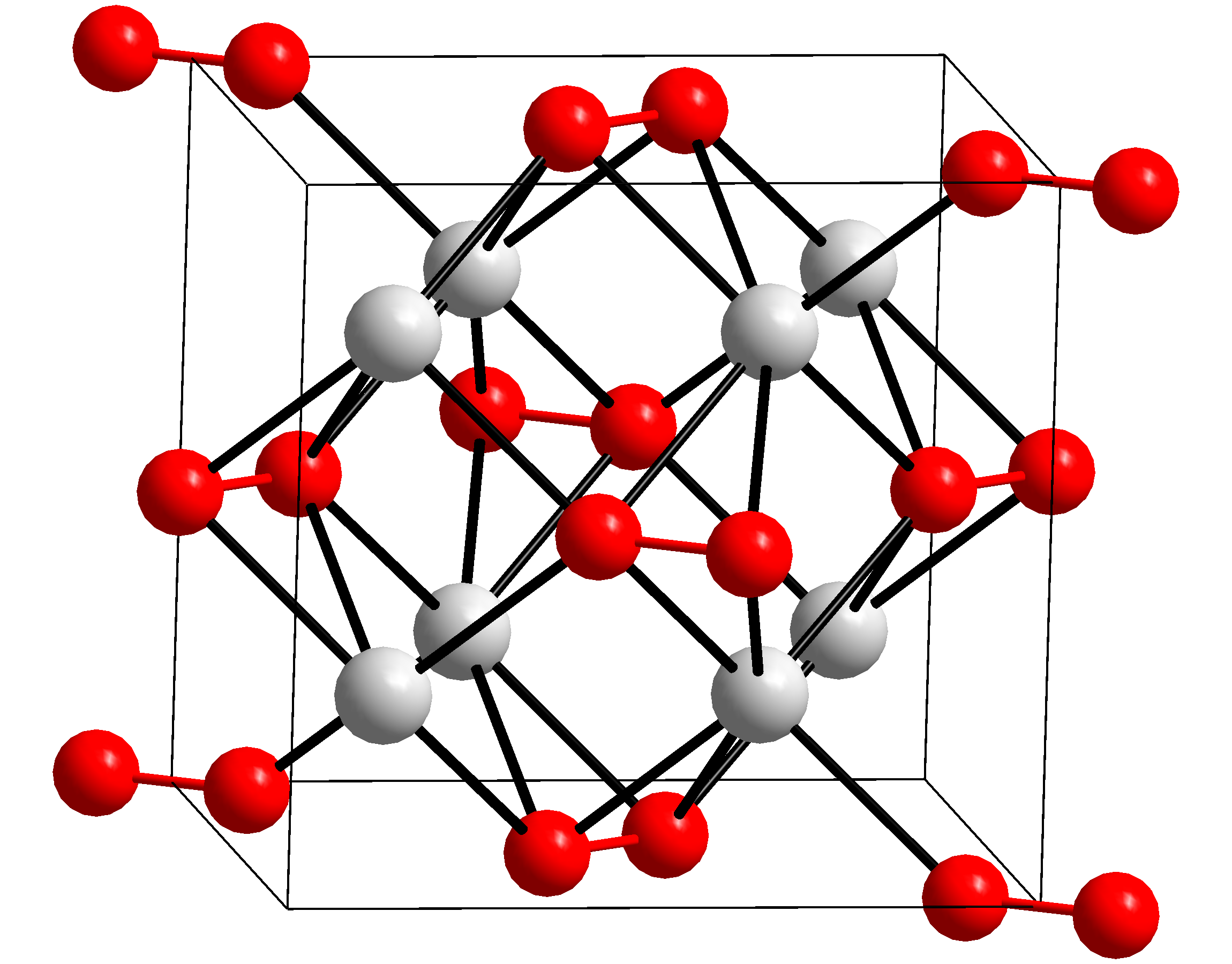
Potash
Potash ( ) includes various mined and manufactured salts that contain potassium in water- soluble form.Potash , USGS 2008 Minerals Yearbook The name derives from ''pot ash'', plant ashes or soaked in water in a pot, the primary means of manufacturing potash before the Industrial Era. The word '''' is derived from ''potash''. Potash is produced worldwide in amounts exceeding 71.9 million |
Potassium Peroxide
Potassium peroxide is an inorganic compound with the molecular formula K2O2. It is formed as potassium reacts with oxygen in the air, along with potassium oxide (K2O) and potassium superoxide (KO2). Potassium peroxide reacts with water to form potassium hydroxide and oxygen: : Properties Potassium peroxide is a highly reactive, oxidizing white to yellowish solid which, while not flammable itself, reacts violently with flammable materials. It decomposes violently on contact with water. The standard enthalpy of formation of potassium peroxide is ΔH f 0 = −496 kJ/mol. Usage Potassium peroxide is used as an oxidizing agent and bleach (due to the peroxide In chemistry, peroxides are a group of Chemical compound, compounds with the structure , where the R's represent a radical (a portion of a complete molecule; not necessarily a free radical) and O's are single oxygen atoms. Oxygen atoms are joined ...), and to purify air. References {{DEFAULTSORT:Potassium Peroxid ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Chemical Element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its atomic nucleus, nucleus. Atoms of the same element can have different numbers of neutrons in their nuclei, known as isotopes of the element. Two or more atoms can combine to form molecules. Some elements form Homonuclear molecule, molecules of atoms of said element only: e.g. atoms of hydrogen (H) form Diatomic molecule, diatomic molecules (H). Chemical compounds are substances made of atoms of different elements; they can have molecular or non-molecular structure. Mixtures are materials containing different chemical substances; that means (in case of molecular substances) that they contain different types of molecules. Atoms of one element can be transformed into atoms of a different element in nuclear reactions, which change an atom's at ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Orthoclase
Orthoclase, or orthoclase feldspar ( endmember formula K Al Si3 O8), is an important tectosilicate mineral which forms igneous rock. The name is from the Ancient Greek for "straight fracture", because its two cleavage planes are at right angles to each other. It is a type of alkali feldspar, also known as potassium feldspar or K-spar. The gem known as moonstone (see below) is largely composed of orthoclase. Formation and subtypes left, Orthoclase Organ_Mountains.html" ;"title="crystal twinning from the Organ Mountains">crystal twinning from the Organ Mountains in New Mexico Orthoclase is a common constituent of most granites and other felsic igneous rocks and often forms huge crystals and masses in pegmatite. Typically, the pure potassium endmember of orthoclase forms a solid solution with albite, the sodium endmember (NaAlSi3O8) of plagioclase. While slowly cooling within the earth, sodium-rich albite lamellae form by exsolution, enriching the remaining orthocla ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Serum (blood)
Serum () is the fluid and solvent component of blood which does not play a role in clotting. It may be defined as blood plasma without the clotting factors, or as blood with all cells and clotting factors removed. Serum contains all proteins except clotting factors (involved in blood clotting), including all electrolytes, antibodies, antigens, hormones; and any exogenous substances (e.g., drugs, microorganisms). Serum also does not contain all the formed elements of blood, which include blood cells, white blood cells ( leukocytes, lymphocytes), red blood cells ( erythrocytes), and platelets. The study of serum is serology. Serum is used in numerous diagnostic tests as well as blood typing. Measuring the concentration of various molecules can be useful for many applications, such as determining the therapeutic index of a drug candidate in a clinical trial. To obtain serum, a blood sample is allowed to clot ( coagulation). The sample is then centrifuged to remove th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrocardiographic
Electrocardiography is the process of producing an electrocardiogram (ECG or EKG), a recording of the heart's electrical activity through repeated cardiac cycles. It is an electrogram of the heart which is a graph of voltage versus time of the electrical activity of the heart using electrodes placed on the skin. These electrodes detect the small electrical changes that are a consequence of cardiac muscle depolarization followed by repolarization during each cardiac cycle (heartbeat). Changes in the normal ECG pattern occur in numerous cardiac abnormalities, including: * Cardiac rhythm disturbances, such as atrial fibrillation and ventricular tachycardia; * Inadequate coronary artery blood flow, such as myocardial ischemia and myocardial infarction; * and electrolyte disturbances, such as hypokalemia. Traditionally, "ECG" usually means a 12-lead ECG taken while lying down as discussed below. However, other devices can record the electrical activity of the heart such as a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioisotope
A radionuclide (radioactive nuclide, radioisotope or radioactive isotope) is a nuclide that has excess numbers of either neutrons or protons, giving it excess nuclear energy, and making it unstable. This excess energy can be used in one of three ways: emitted from the nucleus as gamma radiation; transferred to one of its electrons to release it as a conversion electron; or used to create and emit a new particle (alpha particle or beta particle) from the nucleus. During those processes, the radionuclide is said to undergo radioactive decay. These emissions are considered ionizing radiation because they are energetic enough to liberate an electron from another atom. The radioactive decay can produce a stable nuclide or will sometimes produce a new unstable radionuclide which may undergo further decay. Radioactive decay is a random process at the level of single atoms: it is impossible to predict when one particular atom will decay. However, for a collection of atoms of a single nuc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is considered ''radioactive''. Three of the most common types of decay are Alpha decay, alpha, Beta decay, beta, and Gamma ray, gamma decay. The weak force is the Fundamental interactions, mechanism that is responsible for beta decay, while the other two are governed by the electromagnetic force, electromagnetic and nuclear forces. Radioactive decay is a randomness, random process at the level of single atoms. According to quantum mechanics, quantum theory, it is impossible to predict when a particular atom will decay, regardless of how long the atom has existed. However, for a significant number of identical atoms, the overall decay rate can be expressed as a decay constant or as a half-life. The half-lives of radioactive atoms have a huge range: f ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium-40
Potassium-40 (K) is a long lived and the main naturally occurring radioactive isotope of potassium. Its half-life is 1.25 billion years. It makes up about 0.012% (120 parts-per notation, ppm) of natural potassium. Potassium-40 undergoes four different types of radioactive decay, including all three main types of beta decay: * Electron emission (β) to calcium-40, Ca with a decay energy of 1.31 electronvolt, MeV at 89.6% probability * Positron emission (β) to argon-40, Ar at 0.001% probability * Electron capture (EC) to Ar followed by a gamma decay emitting a photonAlso called a gamma ray, because it is produced by a transition in the nucleus with an energy of 1.46 MeV at 10.3% probability * Direct electron capture (EC) to the ground state of Ar at 0.1%. Both forms of the electron capture decay release further photons,Also called x-ray, as they are emitted from transitions of electrons when electrons from the outer shells fall into the inner shells to replace the electr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemical element), but different nucleon numbers (mass numbers) due to different numbers of neutrons in their nuclei. While all isotopes of a given element have similar chemical properties, they have different atomic masses and physical properties. The term isotope is derived from the Greek roots isos (wikt:ἴσος, ἴσος "equal") and topos (wikt:τόπος, τόπος "place"), meaning "the same place"; thus, the meaning behind the name is that different isotopes of a single element occupy the same position on the periodic table. It was coined by Scottish doctor and writer Margaret Todd (doctor), Margaret Todd in a 1913 suggestion to the British chemist Frederick Soddy, who popularized the term. The number of protons within the atomic nuc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrolysis
In chemistry and manufacturing, electrolysis is a technique that uses Direct current, direct electric current (DC) to drive an otherwise non-spontaneous chemical reaction. Electrolysis is commercially important as a stage in the separation of chemical element, elements from naturally occurring sources such as ores using an electrolytic cell. The voltage that is needed for electrolysis to occur is called the decomposition potential. The word "lysis" means to separate or break, so in terms, electrolysis would mean "breakdown via electricity." Etymology The word "electrolysis" was introduced by Michael Faraday in 1834, using the Greek language, Greek words "amber", which since the 17th century was associated with electrical phenomena, and ' meaning "dissolution". Nevertheless, electrolysis, as a tool to study chemical reactions and obtain pure chemical element, elements, precedes the coinage of the term and formal description by Faraday. History In the early nineteenth century, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |