|
Isolysergic Acid Diethylamide
Iso-LSD, also known as ''d''-iso-LSD, (+)-iso-LSD, or (5''R''-8''S'')-LSD, as well as ''N'',''N''-diethylisolysergamide, is a serotonin receptor modulator of the lysergamide family related to lysergic acid diethylamide (LSD). It is the 8-position epimer of LSD, with iso-LSD being 8α (8''S'') and LSD being 8β (8''R''). Iso-LSD is also the ''N'',''N''-diethyl derivative of isoergine (isolysergic acid amide; iso-LSA), a constituent found in morning glory seeds. Iso-LSD is one of four possible stereoisomers of LSD. Use and effects According to Albert Hofmann and colleagues, iso-LSD is inactive as a psychedelic in humans at doses of up to 500μg, which is up to 25times the minimum given doses of LSD (i.e., 20–50μg).} In other sources, iso-LSD was also stated as being inactive at doses of up to 50μg/kg (3.5mg for a 70-kg person), whereas LSD is active at a dose of 1μg/kg (70μg for a 70-kg person). Hence, iso-LSD is inactive in humans at doses of up to 50times those of a common p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oral Administration
Oral administration is a route of administration whereby a substance is taken through the Human mouth, mouth, swallowed, and then processed via the digestive system. This is a common route of administration for many medications. Oral administration can be easier and less painful than other routes of administration, such as Injection (medicine), injection. However, the onset of action is relatively low, and the effectiveness is reduced if it is not absorbed properly in the digestive system, or if it is broken down by digestive enzymes before it can reach the bloodstream. Some medications may cause gastrointestinal side effects, such as nausea or vomiting, when taken orally. Oral administration can also only be applied to conscious patients, and patients able to swallow. Terminology ''Per os'' (; ''P.O.'') is an adverbial phrase meaning literally from Latin "through the mouth" or "by mouth". The expression is used in medicine to describe a treatment that is taken orally (but not ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Affinity (pharmacology)
In biochemistry and pharmacology, a ligand is a substance that forms a complex with a biomolecule to serve a biological purpose. The etymology stems from Latin ''ligare'', which means 'to bind'. In protein-ligand binding, the ligand is usually a molecule which produces a signal by binding to a site on a target protein. The binding typically results in a change of conformational isomerism (conformation) of the target protein. In DNA-ligand binding studies, the ligand can be a small molecule, ion, or protein which binds to the DNA double helix. The relationship between ligand and binding partner is a function of charge, hydrophobicity, and molecular structure. Binding occurs by intermolecular forces, such as ionic bonds, hydrogen bonds and Van der Waals forces. The association or docking is actually reversible through dissociation. Measurably irreversible covalent bonding between a ligand and target molecule is atypical in biological systems. In contrast to the definition o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ergoline
Ergoline is a core structure in many alkaloids and their synthetic derivatives. Ergoline alkaloids were first characterized in ergot. Some of these are implicated in the condition of ergotism, which can take a convulsive form or a gangrenous form. Even so, many ergoline alkaloids have been found to be clinically useful. Annual world production of ergot alkaloids has been estimated at 5,000–8,000 kg of all ergopeptines and 10,000–15,000 kg of lysergic acid, used primarily in the manufacture of semi-synthetic derivatives. Others, such as lysergic acid diethylamide, better known as LSD, a Semisynthesis, semi-synthetic derivative, and ergine, a natural derivative found in ''Argyreia nervosa'', ''Ipomoea tricolor'' and related species, are known Psychedelic drug, psychedelic substances. Natural occurrence Ergoline alkaloids are found in fungi such as Claviceps purpurea, Claviceps paspali, and the related Periglandula, which have a permanent, symbiotic bond with numerous ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lysergide Stereoisomers Structural Formulae V
Lysergic acid diethylamide, commonly known as LSD (from German ; often referred to as acid or lucy), is a semisynthetic, hallucinogenic compound derived from ergot, known for its powerful psychological effects and serotonergic activity. It was historically significant in psychiatry and 1960s counterculture; it is currently legally restricted but experiencing renewed scientific interest and increasing use. When taken orally, LSD has an onset of action within 0.4 to 1.0 hours (range: 0.1–1.8 hours) and a duration of effect lasting 7 to 12 hours (range: 4–22 hours). It is commonly administered via tabs of blotter paper. LSD is extremely potent, with noticeable effects at doses as low as 20 micrograms and is sometimes taken in much smaller amounts for microdosing. Yet no fatal human overdoses have been documented. LSD is mainly used recreationally or for spiritual purposes. LSD can cause mystical experiences. LSD exerts its effects primarily through high-affinity binding to ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Elimination Half-life
Biological half-life (elimination half-life, pharmacological half-life) is the time taken for concentration of a biological substance (such as a medication) to decrease from its maximum concentration ( Cmax) to half of Cmax in the blood plasma. It is denoted by the abbreviation t_. This is used to measure the removal of things such as metabolites, drugs, and signalling molecules from the body. Typically, the biological half-life refers to the body's natural detoxification (cleansing) through liver metabolism and through the excretion of the measured substance through the kidneys and intestines. This concept is used when the rate of removal is roughly exponential. In a medical context, half-life explicitly describes the time it takes for the blood plasma concentration of a substance to halve (''plasma half-life'') its steady-state when circulating in the full blood of an organism. This measurement is useful in medicine, pharmacology and pharmacokinetics because it helps dete ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
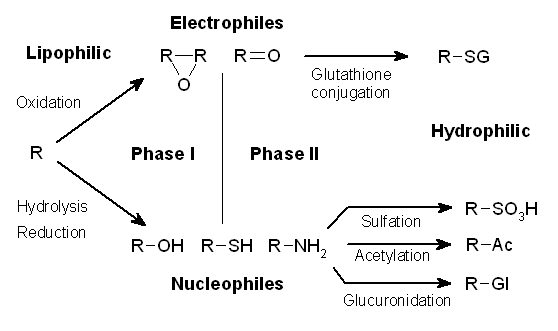
Drug Metabolism
Drug metabolism is the metabolic breakdown of drugs by living organisms, usually through specialized enzymatic systems. More generally, xenobiotic metabolism (from the Greek xenos "stranger" and biotic "related to living beings") is the set of metabolic pathways that modify the chemical structure of xenobiotics, which are compounds foreign to an organism's normal biochemistry, such as any drug or poison. These pathways are a form of biotransformation present in all major groups of organisms and are considered to be of ancient origin. These reactions often act to detoxify poisonous compounds (although in some cases the intermediates in xenobiotic metabolism can themselves cause toxic effects). The study of drug metabolism is the object of pharmacokinetics. Metabolism is one of the stages (see ADME) of the drug's transit through the body that involves the breakdown of the drug so that it can be excreted by the body. The metabolism of pharmaceutical drugs is an important as ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hyperthermia
Hyperthermia, also known as overheating, is a condition in which an individual's body temperature is elevated beyond normal due to failed thermoregulation. The person's body produces or absorbs more heat than it dissipates. When extreme temperature elevation occurs, it becomes a medical emergency requiring immediate treatment to prevent disability or death. Almost half a million deaths are recorded every year from hyperthermia. The most common causes include heat stroke and adverse reactions to drugs. Heat stroke is an acute temperature elevation caused by exposure to excessive heat, or combination of heat and humidity, that overwhelms the heat-regulating mechanisms of the body. The latter is a relatively rare side effect of many drugs, particularly those that affect the central nervous system. Malignant hyperthermia is a rare complication of some types of general anesthesia. Hyperthermia can also be caused by a traumatic brain injury. Hyperthermia differs from feve ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |