|
Internal Pressure
Internal pressure is a measure of how the internal energy of a system changes when it expands or contracts at constant temperature. It has the same dimensions as pressure, the SI unit of which is the pascal. Internal pressure is usually given the symbol \pi_T. It is defined as a partial derivative of internal energy with respect to volume at constant temperature: : \pi _T = \left ( \frac \right )_T Thermodynamic equation of state Internal pressure can be expressed in terms of temperature, pressure and their mutual dependence: :\pi_T = T \left ( \frac \right )_V - p This equation is one of the simplest thermodynamic equations. More precisely, it is a thermodynamic property relation, since it holds true for any system and connects the equation of state to one or more thermodynamic energy properties. Here we refer to it as a "thermodynamic equation of state." Derivation of the thermodynamic equation of state The fundamental thermodynamic equation states for the exact differ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Internal Energy
The internal energy of a thermodynamic system is the energy of the system as a state function, measured as the quantity of energy necessary to bring the system from its standard internal state to its present internal state of interest, accounting for the gains and losses of energy due to changes in its internal state, including such quantities as magnetization. It excludes the kinetic energy of motion of the system as a whole and the potential energy of position of the system as a whole, with respect to its surroundings and external force fields. It includes the thermal energy, ''i.e.'', the constituent particles' kinetic energies of motion relative to the motion of the system as a whole. Without a thermodynamic process, the internal energy of an isolated system cannot change, as expressed in the law of conservation of energy, a foundation of the first law of thermodynamics. The notion has been introduced to describe the systems characterized by temperature variations, te ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Internal Pressure Gases
Internal may refer to: *Internality as a concept in behavioural economics *Neijia, internal styles of Chinese martial arts *Neigong or "internal skills", a type of exercise in meditation associated with Daoism * ''Internal'' (album) by Safia, 2016 See also * *Internals (other) Internals usually refers to the internal parts of a machine, organism or other entity; or to the inner workings of a process. More specifically, internals may refer to: *the internal organs *the gastrointestinal tract The gastrointestinal tra ... * External (other) {{disambig ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peter Atkins
Peter William Atkins (born 10 August 1940) is an English chemist and a Fellow of Lincoln College at the University of Oxford. He retired in 2007. He is a prolific writer of popular chemistry textbooks, including ''Physical Chemistry'', ''Inorganic Chemistry'', and ''Molecular Quantum Mechanics''. Atkins is also the author of a number of popular science books, including ''Atkins' Molecules'', ''Galileo's Finger: The Ten Great Ideas of Science'' and ''On Being''. Career Atkins left school ( Dr Challoner's Grammar School, Amersham) at fifteen and took a job at Monsanto as a laboratory assistant. He studied for A-levels by himself and gained a place, following a last-minute interview, at the University of Leicester. Atkins studied chemistry there, obtaining a BSc degree in chemistry, and a PhD degree in 1964 for research into electron spin resonance spectroscopy, and other aspects of theoretical chemistry. Atkins then took a postdoctoral position at UCLA as a Harkness Fell ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Carbon Dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at normally-encountered concentrations it is odorless. As the source of carbon in the carbon cycle, atmospheric is the primary carbon source for life on Earth. In the air, carbon dioxide is transparent to visible light but absorbs infrared, infrared radiation, acting as a greenhouse gas. Carbon dioxide is soluble in water and is found in groundwater, lakes, ice caps, and seawater. It is a trace gas Carbon dioxide in Earth's atmosphere, in Earth's atmosphere at 421 parts per million (ppm), or about 0.042% (as of May 2022) having risen from pre-industrial levels of 280 ppm or about 0.028%. Burning fossil fuels is the main cause of these increased concentrations, which are the primary cause of climate change.IPCC (2022Summary for pol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Francis Sears
Francis Weston Sears (October 1, 1898 – November 12, 1975) was an American physicist. He was a professor of physics at MIT for 35 years before moving to Dartmouth College in 1956. At Dartmouth, Sears was the Appleton Professor of Physics. He is best known for co-authoring '' University Physics'', an introductory physics textbook, with Mark Zemansky. The book, first published in 1949, is often referred to as "''Sears and Zemansky''", although Hugh Young became a coauthor in 1973. In 1932 he collaborated with Peter Debye in the discovery of what is now called the Debye–Sears effect, the diffraction of light by ultrasonic waves. Sears was a fellow of the Optical Society of America, and was active in the American Association of Physics Teachers, serving as its treasurer from 1950 to 1958, followed by successive one-year terms as president-elect and president. He retired to Norwich, Vermont and died in Hanover, New Hampshire Hanover is a New England town, town located ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Frederick G
Frederick may refer to: People * Frederick (given name), the name Given name Nobility = Anhalt-Harzgerode = *Frederick, Prince of Anhalt-Harzgerode (1613–1670) = Austria = * Frederick I, Duke of Austria (Babenberg), Duke of Austria from 1195 to 1198 * Frederick II, Duke of Austria (1219–1246), last Duke of Austria from the Babenberg dynasty * Frederick the Fair (Frederick I of Austria (Habsburg), 1286–1330), Duke of Austria and King of the Romans = Baden = * Frederick I, Grand Duke of Baden (1826–1907), Grand Duke of Baden * Frederick II, Grand Duke of Baden (1857–1928), Grand Duke of Baden = Bohemia = * Frederick, Duke of Bohemia (died 1189), Duke of Olomouc and Bohemia = Britain = * Frederick, Prince of Wales (1707–1751), eldest son of King George II of Great Britain = Brandenburg/Prussia = * Frederick I, Elector of Brandenburg (1371–1440), also known as Frederick VI, Burgrave of Nuremberg * Frederick II, Elector of Brandenburg (1413–1470), Margrave of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known living organisms (in which it acts as a solvent). It is vital for all known forms of life, despite not providing food energy or organic micronutrients. Its chemical formula, , indicates that each of its molecules contains one oxygen and two hydrogen atoms, connected by covalent bonds. The hydrogen atoms are attached to the oxygen atom at an angle of 104.45°. In liquid form, is also called "water" at standard temperature and pressure. Because Earth's environment is relatively close to water's triple point, water exists on Earth as a solid, a liquid, and a gas. It forms precipitation in the form of rain and aerosols in the form of fog. Clouds consist of suspended droplets of water and ice, its solid state. When finely divided, crystalline ice ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Specific Heat Capacity
In thermodynamics, the specific heat capacity (symbol ) of a substance is the amount of heat that must be added to one unit of mass of the substance in order to cause an increase of one unit in temperature. It is also referred to as massic heat capacity or as the specific heat. More formally it is the heat capacity of a sample of the substance divided by the mass of the sample. The SI unit of specific heat capacity is joule per kelvin per kilogram, J⋅kg−1⋅K−1. For example, the heat required to raise the temperature of of water by is , so the specific heat capacity of water is . Specific heat capacity often varies with temperature, and is different for each state of matter. Liquid water has one of the highest specific heat capacities among common substances, about at 20 °C; but that of ice, just below 0 °C, is only . The specific heat capacities of iron, granite, and hydrogen gas are about 449 J⋅kg−1⋅K−1, 790 J⋅kg−1⋅K−1, and 143 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
James Joule
James Prescott Joule (; 24 December 1818 11 October 1889) was an English physicist. Joule studied the nature of heat and discovered its relationship to mechanical work. This led to the law of conservation of energy, which in turn led to the development of the first law of thermodynamics. The International System of Units, SI unit of energy, the joule (J), is named after him. He worked with Lord Kelvin to develop an absolute thermodynamic temperature scale, which came to be called the Kelvin scale. Joule also made observations of magnetostriction, and he found the relationship between the electric current, current through a resistor and the heat dissipation, dissipated, which is also called Joule's first law. His experiments about energy transformations were first published in 1843. Early years James Joule was born in 1818, the son of Benjamin Joule (1784–1858), a wealthy brewer, and his wife, Alice Prescott, on New Bailey Street in City of Salford, Salford. Joule was tutor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
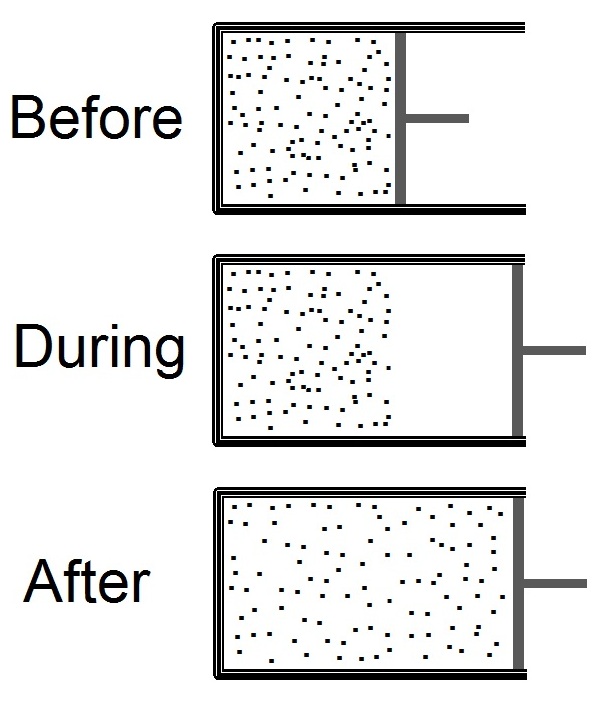
Joule Expansion
The Joule expansion (a subset of free expansion) is an irreversible process in thermodynamics in which a volume of gas is kept in one side of a thermally isolated container (via a small partition), with the other side of the container being evacuated. The partition between the two parts of the container is then opened, and the gas fills the whole container. The Joule expansion, treated as a thought experiment involving ideal gases, is a useful exercise in classical thermodynamics. It provides a convenient example for calculating changes in thermodynamic quantities, including the resulting increase in entropy of the universe (entropy production) that results from this inherently irreversible process. An actual Joule expansion experiment necessarily involves real gases; the temperature change in such a process provides a measure of intermolecular forces. This type of expansion is named after James Prescott Joule who used this expansion, in 1845, in his study for the mechanical e ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Compression Factor
In thermodynamics, the compressibility factor (Z), also known as the compression factor or the gas deviation factor, describes the deviation of a real gas from ideal gas behaviour. It is simply defined as the ratio of the molar volume of a gas to the molar volume of an ideal gas at the same temperature and pressure. It is a useful thermodynamic property for modifying the ideal gas law to account for the real gas behaviour.Properties of Natural Gases . Includes a chart of compressibility factors versus reduced pressure and reduced temperature (on last page of the PDF document) In general, deviation from ideal behaviour becomes more significant the closer a gas is to a |