|
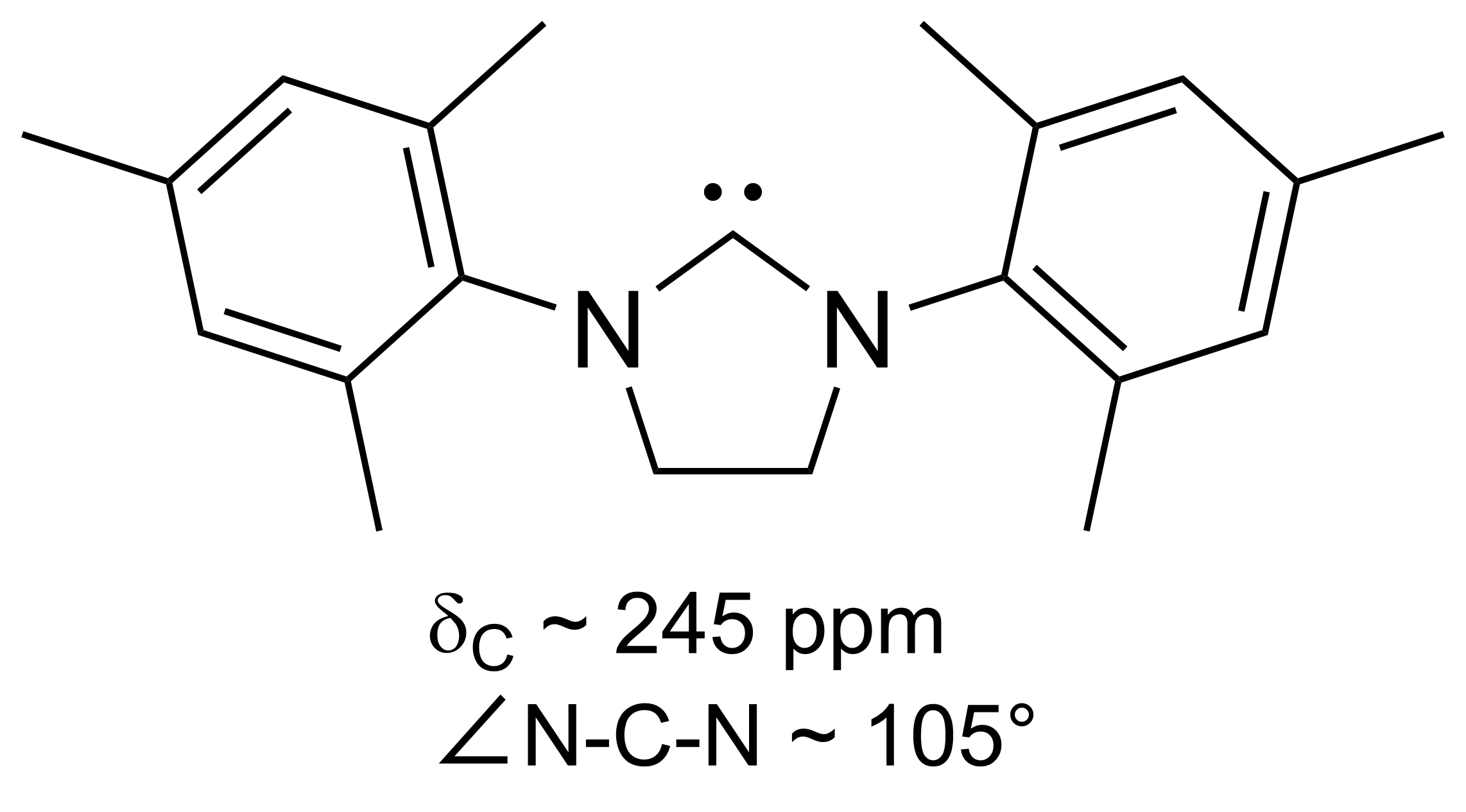
Imes Of
IMes is an abbreviation for an organic compound that is a common ligand in organometallic chemistry. It is an ''N''-heterocyclic carbene (NHC). The compound, a white solid, is often not isolated but instead is generated upon attachment to the metal centre. First prepared by Arduengo, the heterocycle is synthesized by condensation of 2,4,6-trimethylaniline and glyoxal to give the diimine. In the presence of acid, the resulting glyoxal-bis(mesitylimine) condenses with formaldehyde to give the dimesitylimidazolium cation. This cation is the conjugate acid of the NHC. Related compounds Bulkier than IMes is the NHC ligand IPr ( CAS 244187-81-3). IPr features diisopropylphenyl in place of the mesityl substituents. Some variants of IMes and IPr have saturated backbones, two such ligands are SIMes SIMes (or H2Imes) is an N-heterocyclic carbene, ''N''-heterocyclic carbene. It is a white solid that dissolves in organic solvents. The compound is used as a ligand in organometa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organic Compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-containing compounds such as alkanes (e.g. methane ) and its derivatives are universally considered organic, but many others are sometimes considered inorganic, such as certain compounds of carbon with nitrogen and oxygen (e.g. cyanide ion , hydrogen cyanide , chloroformic acid , carbon dioxide , and carbonate ion ). Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. The study of the properties, reactions, and syntheses of organic compounds comprise the discipline known as organic chemistry. For historical reasons, a few classes of carbon-containing compounds (e.g., carbonate salts and cyanide salts), along with a few other exceptions (e.g., carbon dioxide, and even ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Formaldehyde
Formaldehyde ( , ) (systematic name methanal) is an organic compound with the chemical formula and structure , more precisely . The compound is a pungent, colourless gas that polymerises spontaneously into paraformaldehyde. It is stored as aqueous solutions (formalin), which consists mainly of the hydrate CH2(OH)2. It is the simplest of the aldehydes (). As a precursor to many other materials and chemical compounds, in 2006 the global production of formaldehyde was estimated at 12 million tons per year. It is mainly used in the production of industrial resins, e.g., for particle board and coatings. Formaldehyde also occurs naturally. It is derived from the degradation of serine, dimethylglycine, and lipids. Demethylases act by converting N-methyl groups to formaldehyde. Formaldehyde is classified as a group 1 carcinogen and can cause respiratory and skin irritation upon exposure. Forms Formaldehyde is more complicated than many simple carbon compounds in that i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organometallic Chemistry
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, and sometimes broadened to include metalloids like boron, silicon, and selenium, as well. Aside from bonds to organyl fragments or molecules, bonds to 'inorganic' carbon, like carbon monoxide (Metal carbonyl, metal carbonyls), cyanide, or carbide, are generally considered to be organometallic as well. Some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds, though strictly speaking, they are not necessarily organometallic. The related but distinct term "metalorganics, metalorganic compound" refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal β-diketonates, alkoxides, dialkylamides, and metal p ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dibromoethane
Dibromoethane can refer to either of two isomeric organobromides with the molecular formula C2H4Br2: * 1,1-Dibromoethane (ethylidene dibromide) * 1,2-Dibromoethane 1,2-Dibromoethane, also known as ethylene dibromide (EDB), is an organobromine compound with the chemical formula . Although trace amounts occur naturally in the ocean, where it is probably formed by algae and kelp, substantial amounts are produc ... (ethylene dibromide) See also * Dibromoethene {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
SIMes
SIMes (or H2Imes) is an N-heterocyclic carbene, ''N''-heterocyclic carbene. It is a white solid that dissolves in organic solvents. The compound is used as a ligand in organometallic chemistry. It is structurally related to the more common ligand IMes but with a saturated backbone (the S of SIMes indicates a saturated backbone). It is slightly more flexible and is a component in Grubbs' catalyst#Second generation catalyst, Grubbs II. It is prepared by alkylation of trimethylaniline by dibromoethane followed by ring closure and dehydrohalogenation. References [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Conjugate Acid
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid gives a proton () to a base—in other words, it is a base with a hydrogen ion added to it, as it loses a hydrogen ion in the reverse reaction. On the other hand, a conjugate base is what remains after an acid has donated a proton during a chemical reaction. Hence, a conjugate base is a substance formed by the removal of a proton from an acid, as it can gain a hydrogen ion in the reverse reaction. Because some acids can give multiple protons, the conjugate base of an acid may itself be acidic. In summary, this can be represented as the following chemical reaction: \text + \text \; \ce \; \text + \text Johannes Nicolaus Brønsted and Martin Lowry introduced the Brønsted–Lowry theory, which said that any compound that can give a proton to another compound is an acid, and the compound that receives the proton is a base. A proton is a subatomic particle in the n ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Imidazolium
Imidazole (ImH) is an organic compound with the formula . It is a white or colourless solid that is soluble in water, producing a mildly alkaline solution. It can be classified as a heterocycle, specifically as a diazole. Many natural products, especially alkaloids, contain the imidazole ring. These imidazoles share the 1,3-C3N2 ring but feature varied substituents. This ring system is present in important biological building blocks, such as histidine and the related hormone histamine. Many drugs contain an imidazole ring, such as certain antifungal drugs, the nitroimidazole series of antibiotics, and the sedative midazolam. When fused to a pyrimidine ring, it forms a purine, which is the most widely occurring nitrogen-containing heterocycle in nature. The name "imidazole" was coined in 1887 by the German chemist Arthur Rudolf Hantzsch (1857–1935). Structure and properties Imidazole is a planar 5-membered ring, that exists in two equivalent tautomeric forms because hydrogen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glyoxal-bis(mesitylimine)
Glyoxal-bis(mesitylimine) is an organic compound with the formula H2C2(NC6H2Me3)2 (Me = methyl). It is a yellow solid that is soluble in organic solvents. It is classified as a diimine ligand. It is used in coordination chemistry and homogeneous catalysis. It is synthesized by condensation of 2,4,6-Trimethylaniline, 2,4,6-trimethylaniline and glyoxal. In addition to its direct use as a ligand, it is a precursor to imidazole precursors to the popular NHC ligand called IMes. Related compounds *Glyoxal-bis(triisopropylphenylimine), which is bulkier than glyoxal-bis(mesitylimine). {{cite journal, title=Late-Stage Deoxyfluorination of Phenols with PhenoFluorMix, first1=Junting, last1=Chen, first2=Tobias, last2=Ritter, journal=Org. Synth. , year=2019, volume=96, page=16, doi=10.15227/orgsyn.096.0016, doi-access=free References [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's electron pairs, often through Lewis acids and bases, Lewis bases. The nature of metal–ligand bonding can range from covalent bond, covalent to ionic bond, ionic. Furthermore, the metal–ligand bond order can range from one to three. Ligands are viewed as Lewis bases, although rare cases are known to involve Lewis acids and bases, Lewis acidic "ligands". Metals and metalloids are bound to ligands in almost all circumstances, although gaseous "naked" metal ions can be generated in a high vacuum. Ligands in a complex dictate the reactivity (chemistry), reactivity of the central atom, including ligand substitution rates, the reactivity of the ligands themselves, and redox. Ligand selection requires critical consideration in many practical are ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Diimine
Diimines are organic compounds containing two imine (RCH=NR') groups. Common derivatives are 1,2-diimines and 1,3-diimines. These compounds are used as ligands, but they are also precursors to other organic compounds. Preparation Diimines are prepared by condensation reactions where a dialdehyde or diketone is treated with amine and water is eliminated. Many are derived from the condensation of 1,2-diketones and dialdehydes with amines, often anilines. The dialdehyde glyoxal is an especially common precursor. Similar methods are used to prepare Schiff bases and oximes. 1,2-Diimines The 1,2-diimines are also called α-diimines and 1,4-diazabutadienes. An example is glyoxal-bis(mesitylimine), a yellow solid that is synthesized by condensation of 2,4,6-trimethylaniline and glyoxal. 2,2'-Bipyridine is a 1,2-diimine. 1,2-Diketimines are “ non-innocent ligands”, akin to the dithiolenes. : 1,3-Diimines For example, acetylacetone (2,4-pentanedione) and a primary ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Glyoxal
Glyoxal is an organic compound with the chemical formula OCHCHO. It is the smallest dialdehyde (a compound with two aldehyde groups). It is a crystalline solid, white at low temperatures and yellow near the melting point (15 °C). The liquid is yellow, and the vapor is green.O'Neil, M.J. (2001): ''The Merck Index'', 13th Edition, page 803. Pure glyoxal is not commonly encountered because glyoxal is usually handled as a 40% aqueous solution (density near 1.24 g/mL). It forms a series of hydrates, including oligomers. For many purposes, these hydrated oligomers behave equivalently to glyoxal. Glyoxal is produced industrially as a precursor to many products. Production Glyoxal was first prepared and named by the German-British chemist Heinrich Debus (chemist), Heinrich Debus (1824–1915) by reacting ethanol with nitric acid. Commercial glyoxal is prepared either by the gas-phase oxidation of ethylene glycol in the presence of a silver or copper catalyst (the Laporte process) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |