|
Hydroxylamine Oxidoreductase
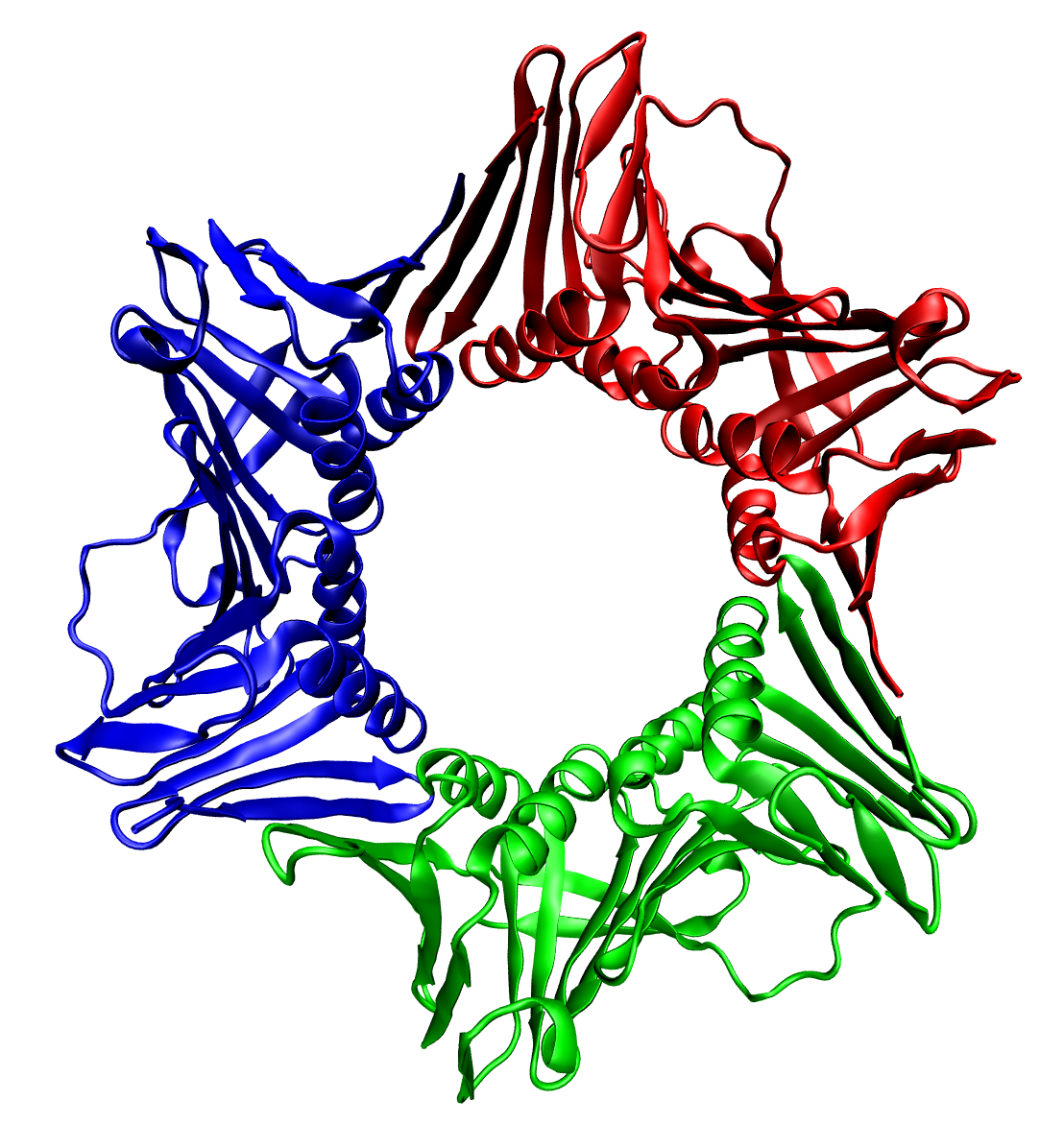
Hydroxylamine oxidoreductase (HAO) is an enzyme found in the prokaryotic genus '' Nitrosomonas.'' It plays a critically important role in the biogeochemical nitrogen cycle as part of the metabolism of ammonia-oxidizing bacteria. The substrate is hydroxylamine (NH2OH), a chemical produced biologically by the enzyme Ammonia monooxygenase. The products of the catalyzed reaction are debated, but recent work shows compelling evidence for the production of nitric oxide. Structural studies Crystallographic methods show that HAO (PDB code: ) is a cross-linked trimer of polypeptides containing 24 heme cofactors. Reactivity For many decades the enzyme was thought to catalyze the following reaction: :NH2OH + H2O -> NO2^- + 5 H+ + 4e^- Recent work in the field, however, reveals that this enzyme catalyzes an entirely different reaction: :NH2OH -> NO + 3H+ + 3e^- Subsequent oxidation of the nitric oxide to nitrite caused by reaction with oxygen accounts for the reactivity previous de ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different molecules known as product (chemistry), products. Almost all metabolism, metabolic processes in the cell (biology), cell need enzyme catalysis in order to occur at rates fast enough to sustain life. Metabolic pathways depend upon enzymes to catalyze individual steps. The study of enzymes is called ''enzymology'' and the field of pseudoenzyme, pseudoenzyme analysis recognizes that during evolution, some enzymes have lost the ability to carry out biological catalysis, which is often reflected in their amino acid sequences and unusual 'pseudocatalytic' properties. Enzymes are known to catalyze more than 5,000 biochemical reaction types. Other biocatalysts include Ribozyme, catalytic RNA molecules, also called ribozymes. They are sometimes descr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrosomonas
''Nitrosomonas'' is a genus of Gram-negative bacteria belonging to the class Betaproteobacteria. It is one of the five genera of ammonia-oxidizing bacteria and, as an obligate chemolithoautotroph, uses ammonia (NH3) as an energy source and carbon dioxide (CO2) as a carbon source in the presence of oxygen. ''Nitrosomonas'' are important in the global biogeochemical nitrogen cycle, since they increase the bioavailability of nitrogen to plants and in the denitrification, which is important for the release of nitrous oxide, a powerful greenhouse gas. This microbe is photophobic, and usually generate a biofilm matrix, or form clumps with other microbes, to avoid light. ''Nitrosomonas'' can be divided into six lineages: the first one includes the species '' Nitrosomonas europea'', '' Nitrosomonas eutropha'', '' Nitrosomonas halophila'', and '' Nitrosomonas mobilis.'' The second lineage presents the species '' Nitrosomonas communis'', ''N. sp. I'' and ''N. sp. II.'' The third line ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrogen Cycle
The nitrogen cycle is the biogeochemical cycle by which nitrogen is converted into multiple chemical forms as it circulates among atmosphere, atmospheric, terrestrial ecosystem, terrestrial, and marine ecosystems. The conversion of nitrogen can be carried out through both biological and physical processes. Important processes in the nitrogen cycle include nitrogen fixation, fixation, ammonification, nitrification, and denitrification. The majority of Earth's atmosphere (78%) is atmospheric nitrogen, making it the largest source of nitrogen. However, atmospheric nitrogen has limited availability for biological use, leading to a scarcity of usable nitrogen in many types of ecosystems. The nitrogen cycle is of particular interest to ecologists because nitrogen availability can affect the rate of key ecosystem processes, including primary production and decomposition. Human activities such as fossil fuel combustion, use of artificial nitrogen fertilizers, and release of nitrogen in w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrifying Bacteria
Nitrifying bacteria are chemolithotrophic organisms that include species of genera such as '' Nitrosomonas'', '' Nitrosococcus'', '' Nitrobacter'', '' Nitrospina'', '' Nitrospira'' and '' Nitrococcus''. These bacteria get their energy from the oxidation of inorganic nitrogen compounds. Types include ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB). Many species of nitrifying bacteria have complex internal membrane systems that are the location for key enzymes in nitrification: ammonia monooxygenase (which oxidizes ammonia to hydroxylamine), hydroxylamine oxidoreductase (which oxidizes hydroxylamine to nitric oxide - which is further oxidized to nitrite by a currently unidentified enzyme), and nitrite oxidoreductase (which oxidizes nitrite to nitrate). Ecology Nitrifying bacteria are present in distinct taxonomical groups and are found in highest numbers where considerable amounts of ammonia are present (such as areas with extensive protein decomposition ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydroxylamine
Hydroxylamine (also known as hydroxyammonia) is an inorganic compound with the chemical formula . The compound exists as hygroscopic colorless crystals.Greenwood and Earnshaw. ''Chemistry of the Elements.'' 2nd Edition. Reed Educational and Professional Publishing Ltd. pp. 431–432. 1997. Hydroxylamine is almost always provided and used as an aqueous solution or more often as one of its salts such as hydroxylammonium sulfate, a water-soluble solid. Hydroxylamine and its salts are consumed almost exclusively to produce Nylon-6. The oxidation of to hydroxylamine is a step in biological nitrification. History Hydroxylamine was first prepared as hydroxylammonium chloride in 1865 by the German chemist Wilhelm Clemens Lossen (1838-1906); he reacted tin and hydrochloric acid in the presence of ethyl nitrate. It was first prepared in pure form in 1891 by the Dutch chemist Lobry de Bruyn and by the French chemist Léon Maurice Crismer (1858-1944). The coordination complex (zin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ammonia Monooxygenase
Ammonia monooxygenase (, ''AMO'') is an enzyme, which catalysis, catalyses the following chemical reaction : ammonia + AH2 + O2 \rightleftharpoons NH2OH + A + H2O Ammonia monooxygenase contains copper and possibly nonheme iron. AMO is the first enzyme in ammonia oxidation. Aerobic oxidation of ammonia to hydroxylamine via AMO is an endergonic reaction. So, all aerobic ammonia oxidizing organisms conserve energy by further oxidizing hydroxylamine. It was believed that aerobic ammonia-oxidizing bacteria oxidize hydroxylamine to nitrite using octahaem hydroxylamine oxidoreductase (HAO). Recently, it was shown that the product of HAO is not nitrite but nitric oxide, which is further oxidized to nitrite by an unknown enzyme. References External links * {{Portal bar, Biology, border=no EC 1.14.99 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst. The rate increase occurs because the catalyst allows the reaction to occur by an alternative mechanism which may be much faster than the noncatalyzed mechanism. However the noncatalyzed mechanism does remain possible, so that the total rate (catalyzed plus noncatalyzed) can only increase in the presence of the catalyst and never decrease. Catalysis may be classified as either homogeneous, whose components are dispersed in the same phase (usual ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitric Oxide
Nitric oxide (nitrogen oxide, nitrogen monooxide, or nitrogen monoxide) is a colorless gas with the formula . It is one of the principal oxides of nitrogen. Nitric oxide is a free radical: it has an unpaired electron, which is sometimes denoted by a dot in its chemical formula (•N=O or •NO). Nitric oxide is also a heteronuclear diatomic molecule, a class of molecules whose study spawned early modern theories of chemical bonding. An important intermediate in industrial chemistry, nitric oxide forms in combustion systems and can be generated by lightning in thunderstorms. In mammals, including humans, nitric oxide is a signaling molecule in many physiological and pathological processes. It was proclaimed the " Molecule of the Year" in 1992. The 1998 Nobel Prize in Physiology or Medicine was awarded for discovering nitric oxide's role as a cardiovascular signalling molecule. Its impact extends beyond biology, with applications in medicine, such as the development of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Trimer
image:4tsv bio r 250.jpg, thumbnail, 400px, Trimeric form of a TNF-α mutant In biochemistry, a protein trimer is a Macromolecule, macromolecular Complex (chemistry), complex formed by three, usually covalent bond, non-covalently bound, macromolecules like proteins or nucleic acids. A protein trimer often occurs from the assembly of a protein's Protein quaternary structure, quaternary structure. The non-covalent interactions between the Hydrophobe, hydrophobic and Hydrophile, hydrophilic regions on the polypeptides units help to stabilize the quaternary structure. Since a protein trimer is composed of multiple polypeptide subunits, it is considered an oligomer. A homotrimer would be formed by three identical molecules. A heterotrimer would be formed by three different macromolecules. Type II Collagen is an example of homotrimeric protein, while Type I collagen is an AAB-type heterotrimeric protein. An example of viral protein homotrimeric protein is mammarenavirus of Z matrix prote ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Heme
Heme (American English), or haem (Commonwealth English, both pronounced /Help:IPA/English, hi:m/ ), is a ring-shaped iron-containing molecule that commonly serves as a Ligand (biochemistry), ligand of various proteins, more notably as a Prosthetic group, component of hemoglobin, which is necessary to bind oxygen in the bloodstream. It is composed of four pyrrole rings with 2 Vinyl group, vinyl and 2 propionic acid side chains. Heme is biosynthesized in both the bone marrow and the liver. Heme plays a critical role in multiple different redox reactions in mammals, due to its ability to carry the oxygen molecule. Reactions include oxidative metabolism (cytochrome c oxidase, succinate dehydrogenase), xenobiotic detoxification via cytochrome P450 pathways (including Drug metabolism, metabolism of some drugs), gas sensing (Guanylate cyclase, guanyl cyclases, nitric oxide synthase), and microRNA processing (DGCR8). Heme is a coordination complex "consisting of an iron ion coordinated ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Greenhouse Gas
Greenhouse gases (GHGs) are the gases in the atmosphere that raise the surface temperature of planets such as the Earth. Unlike other gases, greenhouse gases absorb the radiations that a planet emits, resulting in the greenhouse effect. The Earth is warmed by sunlight, causing its surface to radiate heat, which is then mostly absorbed by greenhouse gases. Without greenhouse gases in the atmosphere, the average temperature of Earth's surface would be about , rather than the present average of .Le Treut, H., R. Somerville, U. Cubasch, Y. Ding, C. Mauritzen, A. Mokssit, T. Peterson and M. Prather, 2007:Chapter 1: Historical Overview of Climate Change. In:Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. olomon, S., D. Qin, M. Manning, Z. Chen, M. Marquis, K.B. Averyt, M. Tignor and H.L. Miller (eds.) Cambridge University Press, Cambridge, United Kingdom and New Y ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitrite
The nitrite polyatomic ion, ion has the chemical formula . Nitrite (mostly sodium nitrite) is widely used throughout chemical and pharmaceutical industries. The nitrite anion is a pervasive intermediate in the nitrogen cycle in nature. The name nitrite also refers to organic compounds having the –ONO group, which are esters of nitrous acid. Production Sodium nitrite is made industrially by passing a mixture of nitrogen oxides into aqueous sodium hydroxide or sodium carbonate solution: : : The product is purified by recrystallization. Alkali metal nitrites are thermally stable up to and beyond their melting point (441 °C for KNO2). Ammonium nitrite can be made from dinitrogen trioxide, N2O3, which is formally the anhydride of nitrous acid: :2 NH3 + H2O + N2O3 → 2 NH4NO2 Structure The nitrite ion has a symmetrical structure (C2v molecular point group, symmetry), with both N–O bonds having equal length and a bond angle of about 115°. In valence bond theory, it is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |